Medigus Ltd. (NASDAQ: MDGS), a medical device company, develops, manufactures, and markets surgical endostaplers and direct vision systems for minimally invasive medical procedures or other commercial use in the United States, Europe, Asia, and internationally. It offers Medigus Ultrasonic Surgical Endostapler, an endoscopy system, which is used for the treatment of gastroesophageal reflux disease. The company also provides miniaturized video cameras for use in various medical procedures, as well as specialized industrial applications. Medigus Ltd. was founded in 1999 and is based in Omer, Israel.
On Wednesday, July 11, 2018, MDGS shares were at $2.10 (+69.35) during intra-day trading. Share volume was over 10 million compared to a daily average of 132 thousand.
On July 9, 2018, the company filed form 6-K with the following information: The Company will effect a reverse split of the Company’s ordinary shares at the ratio of 10:1, such that each ten ordinary shares, par value NIS 0.10 per share, shall be consolidated into one ordinary share, par value NIS 1.00 (the Reverse Split). The record date for determining which holders of the Company’s ordinary shares, and which holders of warrants or options to purchase ordinary shares, will have their holdings adjusted as a result of the Reverse Split will be the close of business on Friday, July 13, 2018.
Read the full 6-K filing here: https://backend.otcmarkets.com/otcapi/company/sec-filings/12853387/content/html
The company’s lead product is the Medigus Ultrasonic Surgical Endostapler, or MUSE™ system, a comprehensive endoscopic device that incorporates the latest technological advancements to deliver a more patient-friendly option for Transoral Fundoplication (TF), the procedure is intended to treat the leading cause of GERD (gastroesophageal reflux disease) — a dysfunctional valve between the esophagus and stomach.
Transoral fundoplication is an incisionless procedure intended to treat GERD. This procedure conducted with the MUSE™ system may provide eligible patients long-term, relief from troublesome reflux symptoms. The MUSE™ System combines the latest microvisual, ultrasonic and surgical stapling capabilities into one device, which allows a single physician to complete the procedure in approximately one hour. Since the MUSE procedure addresses the root cause of the disorder, it has the potential to improve the quality of life for people who suffer from reflux.
Recent Events
June 26, 2018. Medigus announced that they have entered into a development agreement with A.M. Surgical, Inc., an orthopedic company that develops and markets minimally invasive surgical devices for the upper & lower extremity surgeon. The agreement requires Medigus to develop and manufacture an integrated visualization device based on its micro ScoutCam™ technology to work with Stratos™, the A.M. Surgical product for endoscopic procedures. https://finance.yahoo.com/news/medigus-enters-orthopedic-market-development-200500874.html
April 17, 2018. Medigus announced the completion of the first MUSE procedure in Spain. The procedure was performed by Dr. Victor Aguilar at HC Marbella International Hospital. Dr. Aguilar reported that the procedure was successful, and the patient was released from the hospital. https://finance.yahoo.com/news/medigus-hc-marbella-international-hospital-200500343.html
Products
The Medigus Ultrasonic Surgical Endostapler, or MUSE™ system, is a comprehensive endoscopic device that incorporates the latest technological advancements to deliver a more patient-friendly option for Transoral Fundoplication (TF), the procedure is intended to treat the leading cause of GERD (gastroesophageal reflux disease) — a dysfunctional valve between the esophagus and stomach.
They have also developed a range of micro video cameras under their micro ScoutCam™ portfolio of products, including the micro ScoutCam 1.2, which to the best of the company’s knowledge, is the smallest in the world.
Their innovative micro cameras are suitable for both medical and industrial applications and can be customized to meet a range of needs across both applications. They design and manufacture endoscopy and micro camera systems for some of the biggest medical and industrial companies in the world.
About
The Company is a medical device company specializing in developing minimally invasive endosurgical tools and highly innovative imaging solutions. The Company is the developer of the MUSE™ system, an FDA cleared, and CE marked endoscopic device to perform Transoral Fundoplication (TF) for the treatment of GERD (gastroesophageal reflux disease), one of the most common chronic conditions in the world. In 2016, the CMS established the Category I CPT® Code of 43210 for TF procedures, such as the ones performed with MUSE, which establishes reimbursement values for physicians and hospitals. MUSE is gaining adoption in key markets around the world – it is available in world-leading healthcare institutions in the U.S., Europe and Israel. Medigus is also in the process of obtaining regulatory clearance in China.
Financial review
For the three months ended March 31, 2018:
Revenues for the three months ended March 31, 2018, were $67,000, a decrease of $47,000, or 41%, compared to $114,000 for the three months ended March 31, 2017.
Gross loss for the three months ended March 31, 2018, was $16,000, a decrease of $214,000, or 93%, compared to gross loss of $230,000 for the three months ended March 31, 2017.
Sales and marketing expenses for the three months ended March 31, 2018, were $262,000, an increase of $116,000, or 79%, compared to $146,000 for the three months ended March 31, 2017.
General and administrative expenses for the three months ended March 31, 2018, were $383,000, a decrease of $1,006,000, or 72%, compared to $1,389,000 for the three months ended March 31, 2017.
Loss for the three months ended March 31, 2018, was $1,080,000 or $0.01 per basic and diluted ordinary share, compared to approximately $2.0 million or $0.04 per basic and diluted ordinary share in the three months ended March 31, 2017.
Net cash used in operating activities was $1.3 million for the three months ended March 31, 2018, compared to net cash used in operating activities of $1.1 million for the corresponding 2017 period.
Cash and cash equivalents and short-term deposit the Company held as of March 31, 2018 were $5.0 million, compared to $6.3 million as of December 31, 2017.
Stock influences and risk factors
The commercial success of the MUSE™ system or any future product, if approved, could be a catalyst for the company shares;
They will need additional funding. If they are unable to raise capital, they will be forced to reduce or eliminate operations;
Reliance on third-party suppliers for most of the components of their products could harm the ability to meet demand for their products in a timely and cost-effective manner;
They are currently required by the FDA to refrain from using certain terms to label and market their products, which could harm their ability to market and commercialize current or future products.
Stock chart
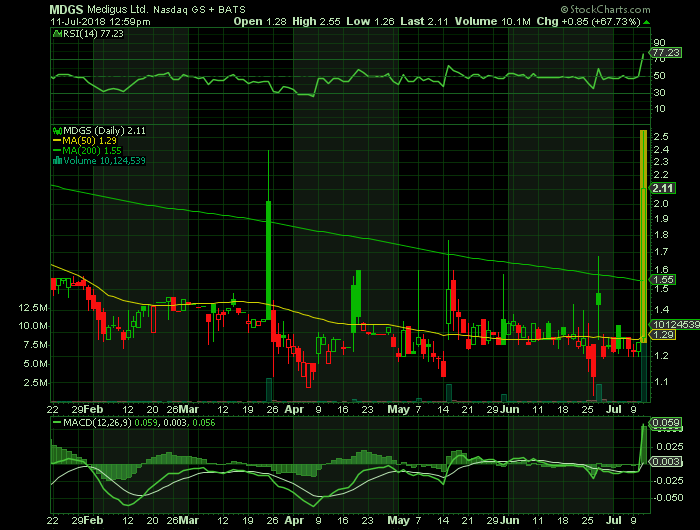
On Wednesday, July 11, 2018, MDGS shares were at $2.11 on traded volume of 10.1 million shares. The current RSI (14) is 77.23
At $2.11, MDGS shares are trading above their 50 DMA and 200 DMA of $1.29 and $1.55 respectively.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

