
Arca Biopharma (NASDAQ: ABIO) up over 150% following our initial report here (updated 01/07/21)
ARCA Biopharma, Inc. (NASDAQ: ABIO), is a biopharmaceutical company, focuses on developing genetically-targeted therapies for cardiovascular diseases. Its lead product candidate is Gencaro, a pharmacologically beta-blocker and mild vasodilator, which is in clinical trial for the treatment of atrial fibrillation in chronic heart failure patients with reduced left ventricular ejection fraction.
ARCA and Medtronic, Inc. have entered into a collaboration to support GENETIC-AF, ARCA’s Phase 2B clinical trial evaluating Gencaro as a potential treatment for atrial fibrillation. Medtronic uses its proprietary CareLink System to collect and analyze the cardiac rhythm data from the implanted Medtronic devices of a substudy of patients participating in GENETIC-AF. Medtronic is the world’s largest medical technology company and a leader in medical technologies to improve the treatment of chronic diseases, including cardiac rhythm disorders.
Recent Events
November 16, 2017. ARCA announced the European Patent Office’s issuance of a patent (EPO # 2515899) on methods of treating cardiovascular disease and conditions with a thiol-substituted isosorbide mononitrate based on genetic targeting. The European patent, entitled “Methods and Compositions for Cardiovascular Diseases and Conditions,” provides protection for this novel approach to treating patients with cardiovascular disease and conditions. The European patent has been validated in ten countries: Denmark, France, Germany, Ireland, Italy, Netherlands, Spain, Sweden, Switzerland, and the United Kingdom. ARCA has related patent applications pending in the United States Patent Office and Canadian Intellectual Property Office.
Products
The ABIO lead product candidate, Gencaro™ (bucindolol hydrochloride), is a pharmacologically unique beta-blocker and mild vasodilator the company is evaluating in a clinical trial for the treatment and prevention of recurrent atrial fibrillation, or AF, in patients with heart failure with reduced left ventricular ejection fraction, or HFREF.
Beta-blockers, a well characterized class of drugs, target cardiac myocytes to reduce adverse beta1- adrenergic signaling that causes cardiac chamber remodeling. Gencaro’s mechanism of action (MOA) is unique among beta-blockers due to its sympatholytic (norepinephrine lowering) and inverse agonism (inactivation of constitutively active receptors) properties.
ABIO has identified common genetic variations in receptors in the cardiovascular system they believe interact with Gencaro’s pharmacology and may predict patient response to the drug. The genotype which responds most favorably to Gencaro, beta-1 389 arginine homozygous, is present in approximately 50% of the U.S. population.
PHASE 2B TOP-LINE DATA ANTICIPATED LATE IN FIRST QUARTER OF 2018
About
ARCA biopharma is dedicated to developing genetically-targeted therapies for cardiovascular diseases through a precision medicine approach to drug development. ARCA’s lead product candidate, GencaroTM (bucindolol hydrochloride), is an investigational, pharmacologically unique beta-blocker and mild vasodilator being developed for the potential treatment of patients with atrial fibrillation and HFrEF, currently in a Phase 2B clinical trial. ARCA has identified common genetic variations that it believes predict individual patient response to Gencaro, giving it the potential to be the first genetically-targeted atrial fibrillation prevention treatment. ARCA has a collaboration with Medtronic, Inc. for support of the GENETIC-AF trial. The Gencaro development program has been granted Fast Track designation by the FDA.
Third Quarter 2017 Summary Financial Results
Cash, cash equivalents and marketable securities totaled $16.0 million as of September 30, 2017, compared to $23.5 million as of December 31, 2016. ARCA had approximately 11.75 million outstanding shares of common stock as of September 30, 2017. ARCA believes that its current cash, cash equivalents and marketable securities will be sufficient to fund its operations, at its projected cost structure, through the end of second quarter of 2018.
Research and development (R&D) expenses for the three months ended September 30, 2017 totaled $3.5 million compared to $3.7 million for the corresponding period of 2016, a decrease of approximately $0.2 million. R&D expense for the nine months ended September 30, 2017 totaled $11.2 million compared to $9.2 million for the corresponding period of 2016, an increase of approximately $2.0 million.
General and administrative (G&A) expenses for the three months ended September 30, 2017 were $1.0 million compared to $1.0 million for the corresponding period in 2016. G&A expenses totaled $3.2 million for the nine months ended September 30, 2017 as compared to $3.1 million for the corresponding period in 2016, a net increase of approximately $79,000
Total operating expenses for the three months ended September 30, 2017 were $4.5 million compared to $4.7 million for the corresponding period in 2016. Total operating expenses for the nine months ended September 30, 2017 were $14.4 million compared to $12.3 million for the corresponding period in 2016.
Net loss was $4.4 million, or $0.39 per share, for the third quarter of 2017 compared to $4.7 million, or $0.51 per share, for the third quarter of 2016. Net loss for the nine months ended September 30, 2017 was $14.3 million, or $1.43 per share, compared to $12.2 million, or $1.35 per share, for the corresponding period in 2016.
Stock Influences and Risk Factors
Any positive new from the FDA would be a significant catalyst
Positive Phase 2B trial data would be a catalyst
If they encounter difficulties enrolling patients in clinical trials, the trials could be delayed or otherwise adversely affected.
If they are not able to develop, obtain FDA approval for, and provide for the commercialization of Gencaro in a timely manner, they may not be able to continue our business operations.
They may not achieve projected development goals in the time frames announced.
Failure to raise substantial additional funding or enter into a strategic transaction may materially and adversely affect the business.
Stock Chart
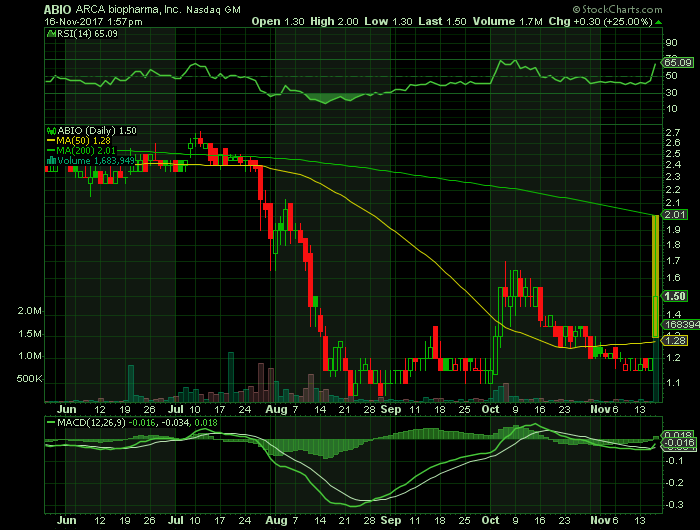
On Thursday, November 16, 2017, ABIO shares were trading at $1.50 (+25%) on traded volume of 1.7 million shares. The current RSI (14) is 65.09
At $1.50, ABIO shares are trading above their 50-day moving average of $1.28 and below their 200-day moving average of $2.01.
Traders News Source Recent Track Record

