Novavax, Inc. NASDAQ: NVAX), is a clinical-stage vaccine company focused on the discovery, development and commercialization of recombinant nanoparticle vaccines and adjuvants. The Company operates through the recombinant vaccines segment. The Company, through its recombinant nanoparticle vaccine technology, produces vaccine candidates to respond to both known and newly emerging diseases.
On September 15, 2016, the company released negative results of its respiratory syncytial virus, or RSV, vaccine trial for older adults. The company’s stock dropped from $7.79/share to $1.29/share on the news and has yet to recover.
RSV is a common source of dangerous infections in both infants and the elderly, leading to an estimated 16,000 deaths and sickening 900,000 among older adults. The company continues to see promise in RSV vaccines, now focusing on its RSV vaccine candidate for infants via maternal immunization. Novavax continues to believe there is a lucrative market opportunity for the RSV-F franchise, and anticipates bringing the first RSV vaccine to market.
The company recently announced financial results and business progress for the 1st quarter. Novavax reported a net loss of $43.9 million, or $0.16 per share, compared to a net loss of $77.3 million, or $0.29 per share, for the first quarter of 2016. Novavax revenue in the first quarter of 2017 increased 35% to $5.7 million, compared to $4.2 million for the same period in 2016, primarily due to increased revenue recorded under the BMGF grant relating to ongoing Prepare clinical trial. Equities analysts predict that Novavax, Inc. will post ($0.62) EPS for the current fiscal year.
As per management, the company continues to make significant progress in the execution of its two key clinical trials of RSV F vaccine for both infant via maternal immunization and in older adults.
Novavax looks forward to reporting important clinical data from its older adult trial in the next 90 days. It has also been in discussion with the FDA about conducting an informational analysis of the Prepare trial that would provide an indication of its vaccine’s potential efficacy. The company believes that it can conduct this analysis in late 2017.
Additionally, management also envisages continued adoption and use of its proprietary adjuvant, Matrix-M, in a number of internal and partnered programs.
Novavax’s products are likely to enhance revenue growth prospects for the company given its product development capabilities and marketing network in different geographies. Notwithstanding NVAX positive growth plan, meaningful & sizeable contribution from the same is partially constrained due to large investment requirements and still evolving commercialization pathway for its product pipeline. Also, the company is likely to face initial integration/gestational issues.
From a stock perspective, NVAX remains in a sweet spot given its promising pipeline potential. Upcoming clinical trial and filing of study documents are the next key triggers.
The following are excerpts of analyst’ outlook on the stock:
Firm: Rating: Price: Date:
Cantor Fitzgerald NEUTRAL $2.00 1-12-2017
FBR & Co. OUTPERFORM $12.00 1-18-2017
Chardan Capital HOLD $1.50 1-18-2017
Seven investment analysts have rated the stock with a hold rating, three have given a buy rating and one has given a strong buy rating to the stock. The stock currently has a consensus rating of “Hold” and a consensus target price of $5.61.
About the company: Novavax is a clinical-stage vaccine company, focuses on the discovery and development of recombinant nanoparticle vaccines and adjuvants. Through its recombinant nanoparticle vaccine technology, it produces vaccine candidates to treat both known and newly emerging diseases. The product pipeline focuses on a range of infectious diseases with vaccine candidates in clinical development for respiratory syncytial virus (RSV), seasonal influenza, pandemic influenza, and the Ebola virus (EBOV).
The lead adjuvant for human applications, Matrix-M, is in clinical trial for pandemic influenza H7N9 vaccine candidate. It is also testing Matrix-M in conjunction with its EBOV vaccine candidate in a clinical trial. It is developing additional pre-clinical stage programs in a range of infectious diseases, including Middle East respiratory syndrome (MERS).
Key highlights for the 1st quarter 2017:
- The company continues enrollment during the second season in the RSV Phase 3 Prepare clinical trial for infants via maternal immunization.
- Enrollment in the first quarter of 2017 expanded to Argentina, Australia, Chile, New Zealand and South Africa. The second season of enrollment has substantially benefitted from the establishment of the operational infrastructure and experience from the first global season, leading to meaningfull increases in enrollment and enhanced momentum as the company moves toward the third global season of enrollment.
- Initiation of a randomized, observer-blinded, multi-arm, dose-ranging Phase 2 clinical trial, in one and two dose formulations, both with and without adjuvants, of its RSV F Vaccine in older adults (60 years of age and older). The trial will assess safety and immunogenicity of these formulations in older adults as measured by serum microneutralization titers against RSV/A and RSV/B, palivizumab competing antibodies (“PCA”) and anti-F IgG.
Upcoming key events/monitorable:
- Initiate a Phase 1 clinical trial of the Company’s recombinant seasonal influenza vaccine candidate before the end of the year.
- Initiate a Phase 1 clinical trial of the Company’s Zika vaccine candidate before the end of the year.
- Announce top-line data from the Phase 2 safety and immunogenicity clinical trial of the RSV F vaccine in older adults in the next 90 days.
- The company also guides it will be filing revised study documents and conduct an informational analysis of the Prepare trial that would provide an indication of the RSV F Vaccine’s potential efficacy against the trial’s primary endpoint before the end of the year.
Q1 2017 Results
Earnings: Novavax revenue in the first quarter of 2017 increased 35% to $5.7 million, compared to $4.2 million for the same period in 2016, primarily due to increased revenue recorded under the BMGF grant relating to ongoing Prepare clinical trial.
Profitability: Novavax reported a net loss of $43.9 million, or $0.16 per share, for the first quarter of 2017, compared to a net loss of $77.3 million, or $0.29 per share, for the first quarter of 2016.
Research and development expenses decreased 45% to $37.7 million in the first quarter of 2017, compared to $69.0 million for the same period in 2016. The decrease was primarily due to reduced costs associated with the clinical trials and development activities of RSV F Vaccine and lower employee-related costs.
General and administrative expenses decreased 16% to $8.9 million in the first quarter of 2017, compared to $10.5 million for the same period in 2016. The decrease was primarily due to lower professional fees for pre-commercialization activities.
Interest income (expense), net for the first quarter of 2017 was ($3.0) million, compared to ($1.9) million for the same period in 2016.
Liquidity: As of March 31, 2017, the company had $211.2 million in cash and cash equivalents and marketable securities compared to $235.5 million as of December 31, 2016. Net cash used in operating activities for the first quarter of 2017 was $38.6 million, compared to $69.8 million for same period in 2016. The decrease in cash usage was primarily due to decreased costs relating to RSV F Vaccine and lower employee-related costs.
Key risk factors and potential stock drivers:
- The company is in the process of obtaining regulatory approvals for its upcoming products. Therefore, any positive announcement related with approvals, outcome of ongoing trials, could be a near term trigger for stock performance.
- Over medium to longer term, successful launch & commercialization of existing and future products would be critical given the considerable investments undertaken in R&D and infrastructure development during the recent past.
- Given high investments incurred related to the marketing products in different markets, cash drainage in its ongoing R&D activities and uncertainties related to the outcome of recent trials, overall return/profitability indicators could remain muted during the initial gestation period.
- The company is likely to generate substantial net losses and negative cash flow from operations over the near to medium term. Therefore, its available cash balance might not be adequate to fund its anticipated level of operations. Therefore, timely arrangement of incremental funding would remain a critical liquidity & financial flexibility factor.
Stock Performance:
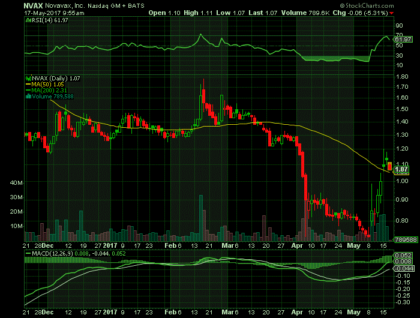
On Tuesday, May 16th, 17, NVAX shares surged to $1.13 on an average volume of 10M shares exchanging hands. The current RSI is 61.97.
In the past 52 weeks, shares of NVAX have traded as low as $0.73 and as high as $8.49
At $1.00, shares of NVAX are trading above its 50-day moving average (MA) at $1.05 and below its 200-day MA at $2.31.
Welcome to Traders News Source Small Cap and Mid Cap Research for the Independent Trader (see our track record below)
Expect 3-4 small cap profiles per month consisting of two emails per week. We do not spam or send emails daily, we understand that is annoying! Our reports are only sent when we see an actionable situation and potential for near term gains.
Traders News Source recent profiles and track record, 534% in verifiable potential gains for our members on three of our well-timed reports alone!
January 31st, 2017 (NASDAQ: HIMX) opened at $5.10/share and hit a high of $9.68/share March 24th, 2017 for gains of 89% within 60 days- http://finance.yahoo.com/news/himax-technologies-review-4q-2016-130000319.html
February 6th, 2017- (NASDAQ: SCON) opened at $1.12/share hit a high of $1.80/share within 10 days our member potential gains- 60% – http://finance.yahoo.com/news/superconductor-technologies-potential-revolutionize-smart-130000844.html
March 6th, 2017 (OTC: USRM) opened at .035/share and hit over .17/share within 25 days for gains of 385% for our members- http://finance.yahoo.com/news/traders-news-issues-comprehensive-report-130000743.html
These are numbers that make traders drool. Any trader in any market would fall all over themselves to see numbers like this. So, if you’ve been on the fence, perhaps it’s time to start doing some research and verify our numbers for yourself. We are constantly raising the bar and separate ourselves from the rest of the small-cap newsletters as the best in business.
We know with a large following comes a large responsibility as we have everyone from institutional investors to the beginner following our profiled securities in our newsletters. This is something we take very seriously always seeking small cap growth companies that have both near and long-term potential for our members.
Limited Time Offer VIP Mobile Alerts
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP – SMS/text alert service for free, simply text the word “Traders” to the phone number “25827” from your cell phone***
Traders News Source Mission Statement
We strive to highlight the future potential as well as the inherent risk in each small cap company we cover while remaining neutral as a leading third-party equity research firm.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

