Therapix Biosciences Ltd. (NASDAQ: TRPX) is a specialty clinical-stage pharmaceutical company creating and enhancing a portfolio of technologies and assets based on cannabinoid pharmaceuticals.
On October 1st, TRPX shares closed at $4.57 and have since then hit an intra-day high of $7.65 (+67%). Trading volumes have increased dramatically in the same time frame.
The cannabinoid system is prone to “entourage effect”, whether THC being potentiated by other cannabinoids or by terpene compounds. Therapix’s leading entourage compound is PEA. Combining PEA with THC may stimulate cannabinoid receptors, inhibit metabolic degradation, and thus increase uptake of THC.
_______________________________________________________________________
Our members have booked up to 800% on our recent NASDAQ and NYSE small cap alerts. We will be initiating coverage on another exciting small cap security soon, the week of 10/08/18. View our recent picks, track record and sign up for our mobile/text alerts in real time here- https://tradersnewssource.com/traders-news-source-new-members/
_______________________________________________________________________
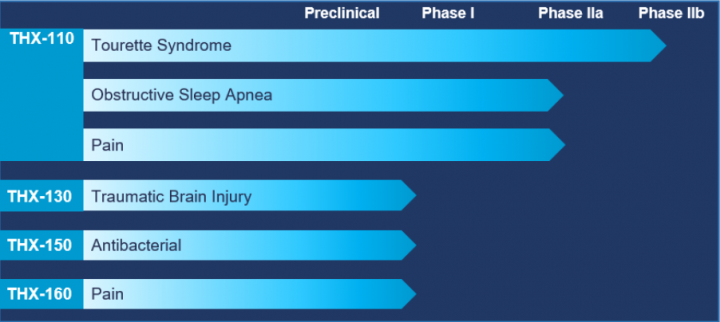
Product Candidates
Four clinical programs to treat underserved central nervous system disorders:
THX-110: A drug candidate platform for the treatment of symptoms related to Tourette syndrome (TS), as well as Obstructive Sleep Apnea (OSA) and chronic pain.
The THX-110 program takes a unique approach to cannabinoid formulation using the “Entourage Effect” hypothesis (i.e. in which Endocannabinoid system constituents work synergistically together). THX-110 is a proprietary combination drug based on dronabinol and PEA. PEA is a cannabinoid mimetic lipid molecule, which naturally occurs in various plant and animal sources.
THX-130: An “Ultra Low-Dose” THC targeting the high value and unserved market of Mild Cognitive Impairment (MCI), using a unique and proprietary drug development and delivery dosage platform.
THX-150: A drug candidate intended for the treatment of infectious diseases. It consists of a selected antibacterial agent, with THC or a combination of THC and PEA, to potentially exert antimicrobial synergy.
THX-160: An innovative and proprietary CB2 receptor agonist formulation intended for the treatment of pain. This specific CB2 receptor agonist was synthesized by Professor Raphael Mechoulam, Ph.D.
THX-110, THX-130 and THX-150 are using a 505 (b)(2) regulatory strategy based on prior FDA approval of dronabinol.
About
Therapix Biosciences Ltd. is a specialty clinical-stage pharmaceutical company led by an experienced team of Senior Executives and Scientists. Our focus is creating and enhancing a portfolio of technologies and assets based on cannabinoid pharmaceuticals. With this focus, the company is currently engaged in the following drug development programs based on repurposing an FDA-approved cannabinoid (Dronabinol): THX-110 for the treatment of Tourette syndrome (TS), for the treatment of Obstructive Sleep Apnea (OSA), and the treatment of pain; THX-130 for the treatment of Mild Cognitive Impairment (MCI) and Traumatic Brain Injury (TBI); THX-150 for the treatment of infectious diseases; and THX-160 for the treatment of pain.
Financial review
Q2 and H1 2018
TRPX is a development stage company and has not generated revenues yet.
Net loss of $1.2 million, or $0.35 per ADS, for the three months ended June 30, 2018, compared to a net loss of $1.86 million, or $1.10 per ADS, for the three months ended June 30, 2017. For the six months ended June 30, 2018, net loss of $3.26 million, or $0.93 per ADS, compared to a net loss of $2.5 million, or $1.07 per ADS, for the comparable period in 2017.
Research and development expenses amounted to $0.65 million for the three months ended June 30, 2018, compared to approximately $0.46 million for the three months ended June 30, 2017. For the six months ended June 30, 2018, R&D expenses amounted to $1.64 million, compared to $0.7 million for the comparable period in 2017.
General and administrative expenses amounted to $1 million for the three months ended June 30, 2018, compared to $0.97 million for the three months ended June 30, 2017. For the six months ended June 30, 2018, G&A expenses amounted to $2.14 million, compared to $1.37 million for the comparable period in 2017.
Cash totaled $5.1 million as of June 30, 2018, compared to $9.2 million as of December 31, 2017.
Stock influences and risk factors
Positive results from the company’s clinical trials could act as a catalyst for the company shares.
They have not generated any revenue from the sale of current product candidates and may never be profitable.
They are subject to numerous complex regulations and failure to comply with these regulations, or the cost of compliance with these regulations, may harm the business.
They will need to raise substantial additional funding before they can expect to become profitable from sales of their product candidates. This additional financing may not be available on acceptable terms, or at all.
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable.
Stock chart
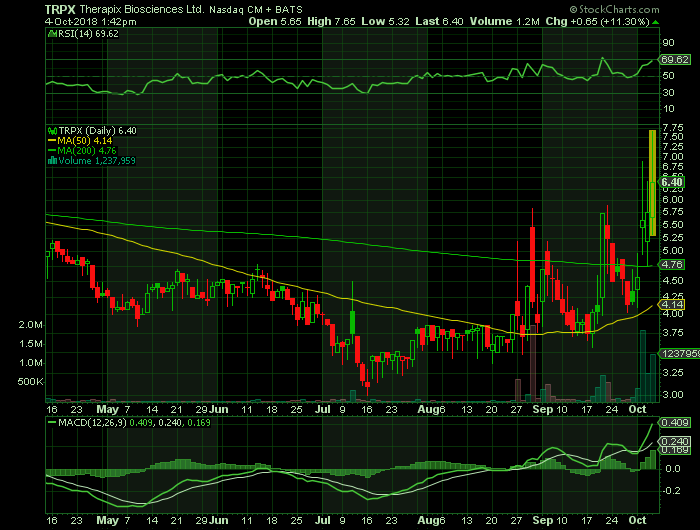
On Thursday, October 4, 2018, TRPX shares were at $6.40 on traded volume of 1.2 million shares. The current RSI (14) is 69.62
At $6.40, TRPX shares are trading above their 50 DMA and 200 DMA of $4.14 and $4.76 respectively.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

