Amedica Corporation (NASDAQ: AMDA) is a commercial biomaterial company focused on using its silicon nitride technology platform to develop, manufacture and sell a broad range of medical devices. Amedica markets spinal fusion products and is developing a new generation of orthopedic bearings for hip and knee arthroplasty. The company operates an ISO 13485 certified manufacturing facility and its spine products are FDA cleared, CE marked, and currently marketed in the U.S. and select markets in Europe and South America.
Recent Events
September 10, 2018. AMDA announced the filing of a U.S. patent application for breakthrough research findings that have identified a new property of its proprietary silicon nitride. Amedica investigators led by Dr. Giuseppe Pezzotti, a professor at the Kyoto Institute of Technology (Japan) and consultant to Amedica Corporation, have developed micromeritic silicon nitride powders as well as bulk surfaces that are effective against several strains of commonly prevalent viruses – including Influenza A virus (H1N1), the virus responsible for the 2009 flu pandemic. https://finance.yahoo.com/news/amedica-announces-filing-key-patent-130000587.html
September 06, 2018. Amedica announced that it has entered into an asset purchase agreement with CTL Medical, a Dallas, TX-based privately held medical device manufacturer that focuses on the spine implant and instrument market, whereby CTL Medical will acquire all of Amedica’s commercial spine business for total consideration of up to $10 million. The transaction is expected to close in the third quarter of 2018, and is subject to usual and customary due diligence and closing conditions. https://finance.yahoo.com/news/amedica-announces-agreement-sell-spine-130000449.html
Products
The silicon nitride biomaterial produced by AMDA is ideal for a wide variety of medical applications including spine, dental, hip, knee, extremities, etc.
Silicon nitride was first prepared in 1857 and was little more than a chemical curiosity for nearly a century. However, things changed in the late 1940s when the compound began to be used across multiple industries, ultimately becoming a key component in electronics, turbo machinery, bearings, and even orbital satellites.
Amedica’s founders discovered that silicon nitride is stronger than bone and PEEK, biologically kind (accepted by the human body) and produced artifact- free imaging. These promising findings led to the development of a proprietary composition of silicon nitride well-suited for medical device applications.
Amedica’s 30,000 sq. ft. ISO 13485 certified manufacturing plant in Salt Lake City, UT is the only medical device production facility in the world that produces CE marked and FDA cleared silicon nitride implants. The operation is vertically integrated from powder processing through to finished product and rigorous quality control. It has the flexibility to produce intricately shaped prototype and production components ranging in size from small dental implants to large articular femoral heads.
_______________________________________________________________________
Our members have booked up to 800% on our recent NASDAQ and NYSE small cap alerts. We will be initiating coverage on another exciting small cap security soon, the week of 10/08/18. View our recent picks, track record and sign up for our mobile/text alerts in real time here- https://tradersnewssource.com/traders-news-source-new-members/
_______________________________________________________________________
About
Amedica Corporation, a biomaterial company, develops, manufactures, and commercializes a range of medical devices based on its silicon nitride technology platform in the United States, Europe, and South America. The company offers silicon nitride implants to surgeons and hospitals for use in cervical and thoracolumbar spine surgery under the Valeo brand. It also provides a line of non-silicon nitride spinal fixation products to address spinal deformity and degenerative conditions. The company markets and sells its products directly; and through a network of independent sales distributors, as well original equipment manufacturer and private label partnerships. Amedica Corporation was founded in 1996 and is headquartered in Salt Lake City, Utah.
Analysts
2 Wall Street analysts have issued ratings and price targets for Amedica in the last 12 months. There are currently 2 hold ratings for the stock, resulting in a consensus rating of “Hold.”
Date Brokerage Action Rating
5/8/2018 Maxim Group Reiterated Rating Hold
11/27/2017 LADENBURG THALM/SH Downgrade Buy ➝ Neutral
Financial review
Q2 2018
Revenue $2,029,000
Gross profit 1,518,000
Expenses 4,072,000
Operating loss 2,033,000
Net loss 2,312,000
Cash 8,835,000
Stock influences and risk factors
Successful commercialization of silicon nitride-based medical devices could be a catalyst for AMDA shares.
The orthopedic market is highly competitive, and they may not be able to compete effectively against the larger, well-established companies that dominate this market.
They have significant customer concentration, so that economic difficulties or changes in the purchasing policies or patterns of key customers could have a significant impact on the business and operating results.
Use of third-party manufacturers increases the risk that they will not have adequate supplies of non-silicon nitride products or instrumentation sets.
Stock chart
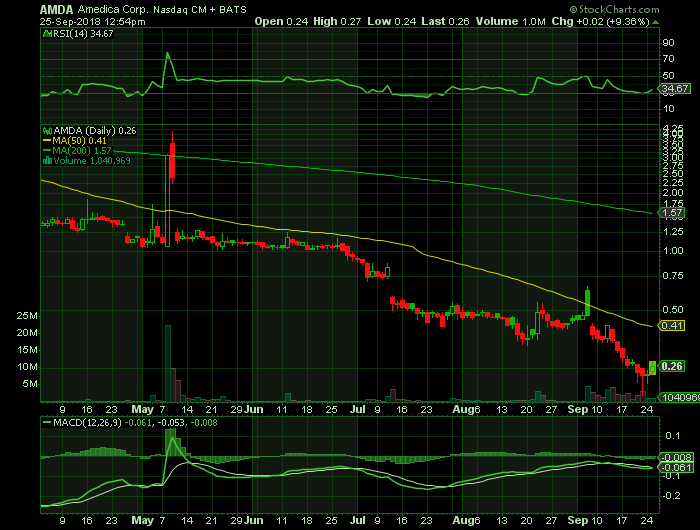
On Tuesday, September 25, 2018, AMDA shares were at $.26 on traded volume of 1.0 million shares. The current RSI (14) is 34.67
At $.26, AMDA is trading below its 50 DMA and 200 DMA of $.41 and $1.57 respectively.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

