Case study: Vagal tone diagnostics with the VAGUS® smartwatch ECG test to detect autonomic nervous system changes triggered by transcutaneous vagus nerve stimulation and oral intake of Famotidine (Pepcid)
Case study of a 74yrs old male with chronic liver and pancreas inflammation.
Author: Dr. Ulf Andersson, Gustaf Kranck, Dr. Fintan Nagle
Published as part of Vagus Health Ltd scientific findings.
Stockholm, Sweden. December 22nd, 2022.
BACKGROUND
The patient is a pediatric rheumatologist, a retired professor from Karolinska Institute, 74 years old, who in parallel to clinical work, performed basic research on neuronal regulation of inflammation. During the last 25 years, the patient has focused on two endogenous molecules named acetylcholine and HMGB1, two central neurotransmitters acting as antagonists in the control of acute and chronic inflammation. Acetylcholine is the effector molecule released by the cholinergic antiinflammatory mechanism operating after vagus nerve stimulation to counteract inflammation and pain. In contrast, HMGB1 released by sensory nerves provokes and supports inflammation and pain. Furthermore, HMGB1 is also a nuclear protein present in every animal cell, expressing no activity connected to inflammation provided that the protein remains in the nucleus. However, any cell which threatens to depart from the homeostatic regime will release HMGB1 extracellularly as an alarm signal to generate inflammation aimed to restore cellular balance.
A remarkable coincidence is that almost two decades ago, the patient fell ill with a severe chronic inflammatory condition driven by HMGB1 and where acetylcholine, released via treatment with transcutaneous auricular vagus nerve stimulation (taVNS) has enabled the patient to return to an active and normal life. Lessons that the patient learnt from this long clinical and scientific voyage, starting long before the presentation of inflammatory disease, inspired the patient to write this case report.
The patient has had no health problems until 20 years ago, when he was diagnosed with a large, non-malignant pancreatic tumor that obstructed the outflow to the gut for both bile and pancreatic fluids. He then went through major abdominal surgery, the Whipple procedure, to remove the head of the pancreas, gallbladder, the outer portion of the bile duct, the duodenum, proximal jejunum, and part of the stomach. This is the standard method to surgically treat pancreatic cancer. Although the tumor was benign, it required this extensive surgical procedure due to its size. An expected but highly unwanted residual state that remains permanently after Whipple surgery is that intestinal bacteria get free access to reach the remaining bile and pancreatic ducts, sites that normally are sterile. The patient thus developed chronic infection and inflammation, like all post-Whipple patients, in the liver and in the residual part of the pancreatic tissue: chronic cholangitis and chronic pancreatitis. These conditions did not cause any severe clinical trouble. The patient’s major problem was that he suffered from flares of acute cholangitis 2- 4 times every year, caused by aggressive gut bacteria. The patient generally managed to handle these dramatic events at home with large doses of antibiotics. The acute stage normally lasted 4 or 5 days, but was followed by months with severe fatigue, reduced cognitive capacity, and above all the appearance of generalized anxiety and depressive symptoms, something that the patient had never encountered before. These central nervous symptoms accumulated and accelerated after each episode of acute cholangitis, and during a period of 8 years the patient was highly dependent on continuous psychiatric support including daily anxiolytic medication with a need for escalating doses each year. This treatment enabled the patient to work effectively in his profession, but he felt that his wings were cut, since he was always tired and did not have access to his full mental resources. Family life suffered badly because of fatigue and subsequent change of life style. The patient has four children, two of whom were rather young then, and it was very hard to manage to act as a vital father and a good husband. The marriage ended with a divorce, connected to these health problems. In 2013, the patient suffered a minor coronary infarction and had a coronary stent implanted. His peak plasma cardiac troponin T value was 21 ng/L, to be compared to an upper reference value of 15 ng/L. A large infarction may generate a plasma cardiac troponin T concentration of several thousand ng/L. The patient was thus lucky to get the treatment early enough to restrict the heart tissue damage.
Subsequently, in 2013, the patient started to take daily doses of antibiotics at reduced levels as a prophylaxis for acute episodes of cholangitis. This helped considerably since the yearly number of flare-ups decreased by half; however, he was still dependent on psychotropic medication, which caused multiple side effects. In 2015, he added daily oral doses of lactoferrin capsules to his treatment with additional beneficial, but still insufficient, effects. Lactoferrin is an endogenous protein that neutralizes a bacterial toxin called lipopolysaccharide (LPS) released by intestinal bacteria, which will be discussed later when explaining the pathogenesis of the patient’s chronic inflammation and his reasons for trying vagus nerve stimulation treatment.
In 2017, the patient started to use transcutaneous auricular VNS (taVNS), aiming to ameliorate his chronic inflammation. This treatment has been a success and has improved quality of life to what the patient experienced 20 years ago before falling ill. The patient stimulated the cymba concha of the left ear 5 min twice every day with a handheld probe comprising two electrodes with carbonized silicone plugs attaching to the skin in the auricle. The TENS unit generating the electrical signals delivers an asymmetrical bi-phasic square pulse with a pulse width of 200 microseconds at 20 Hz. The pulse amplitude during taVNS was varied between 2-4 mA. After having used taVNS for 6 years, the patient is in excellent physical and mental shape. He walks at a good pace in the forest for at least 1 hour every day and his medication is restricted to amoxicillin (antibiotics) in prophylactic doses and lactoferrin capsules. There has been no need for psychotropic drugs throughout the last 5 years.
For the last two years, the patient has had access at home to a Vagus ECG Smartwatch developed by Vagus Health Ltd, Cambridge, UK. The patient has used the device regularly to monitor cardiac parameters recorded during controlled breathing, and has made ECG recordings before, during, and after taVNS sessions. During the last 6 months, the patient has performed approximately 500 recordings. The generated results are displayed via a smartphone app, both numerically and by means of graphical visualisations. Each recording provides information about heart rate, heart rate variability (HRV) expressed as the standard deviation of the average normal-to-normal intervals (SDNN), and synchronization between HRV and controlled breathing (respiratory sinus arrhythmia or RSA, and RSA synchronization i.e. the RSA Sync measure).
Recordings have shown highly consistent results. Before taVNS, the patient has a heart rate around 60 beats per minute with a sinus rhythm and HRV(SDNN) shows average scores, while the synchronization between heart rate changes during controlled breathing pattern, for unknown reasons, is extremely poor (Figs 1-4).
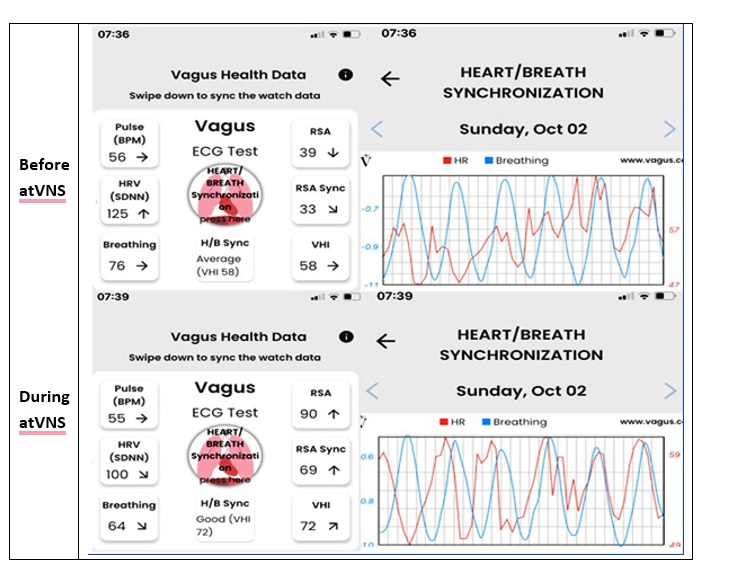
Fig 1. Typical recordings with the Vagus ECG Smartwatch performed before and during taVNS. The chaotic synchronization between heart rate variability and the breathing pattern is apparent before taVNS (upper images) and fully normalized during taVNS (lower images).
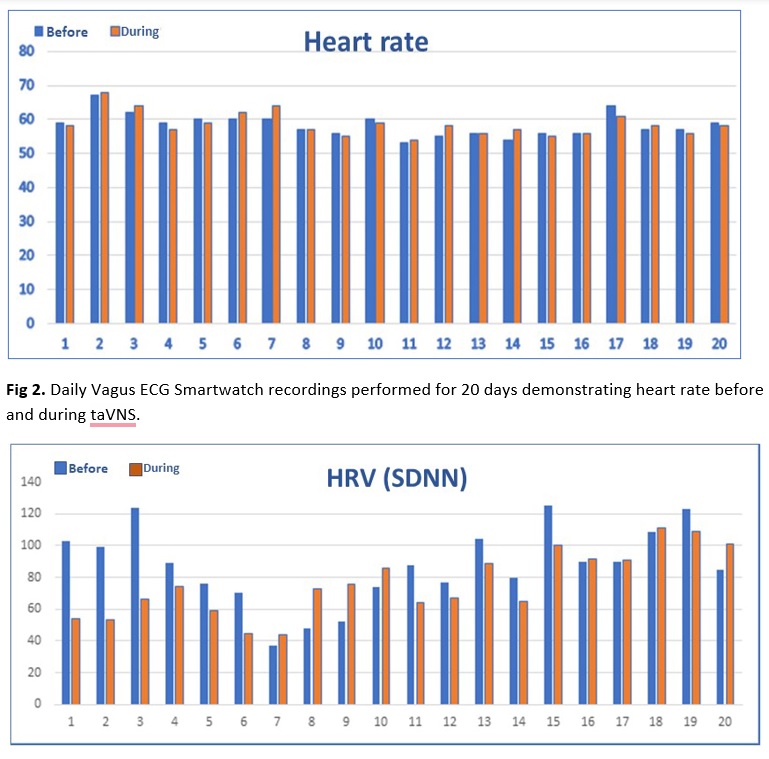
Fig 3. DailyVagus ECG Smartwatch recordings performed for 20 days demonstrating heart rate variability (HRV-SDNN) before and during taVNS with no consistent changes.
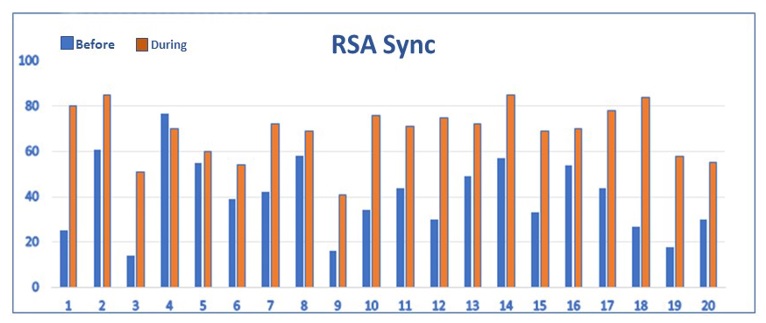
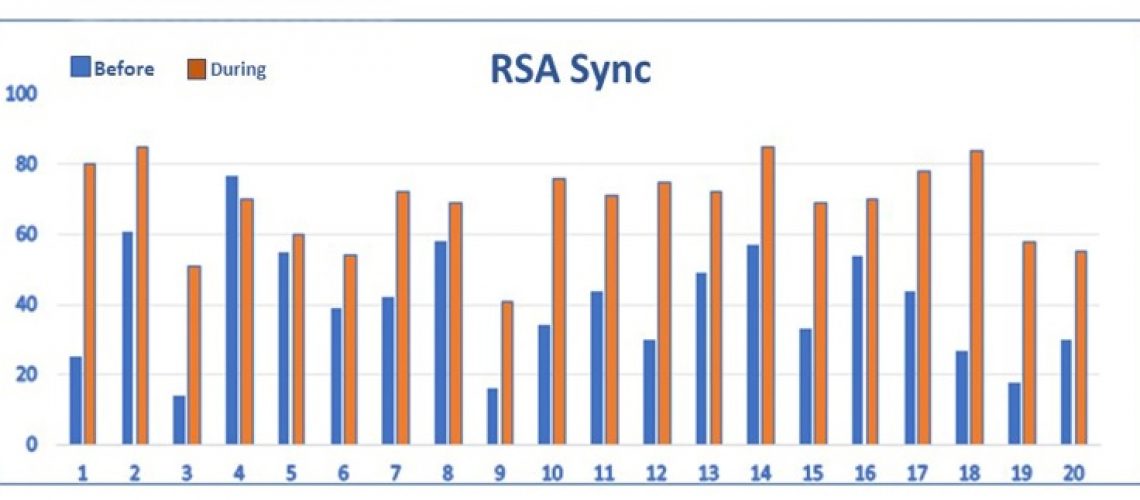
Fig 4. DailyVagus ECG Smartwatch recordings performed for 20 days demonstrating RSA Sync= Respiratory Sinus Arrythmia Synchronization Index (0-100) before and during taVNS. The RSA Sync results improved during taVNS on 19 out of 20 days indicating improved synchronization of cardiac and respiratory activities.
The patient has regularly visited a cardiologist after his coronary infarction in 2013, and the outcomes of these yearly examinations with ECG at rest and during exercise are free from pathological findings. The results of Vagus ECG Smartwatch recordings during taVNS are strikingly different than the scores seen before taVNS, since the synchronization between cardiac and respiratory performance shows normal results at each test. In contrast, there are no consistent findings regarding change of pulse and HRV(SDNN) results during taVNS compared to before taVNS. Another interesting observation is that oral intake of 60 mg famotidine (Pepcid) instead of taVNS produces identical results to those seen after taVNS (Fig 5). The low levels of synchronization observed before taVNS or before Pepcid intake is improved to a normal pattern after Pepcid administration. Pepcid is a histamine receptor antagonist used clinically to reduce gastric acid production. However, this is not the mechanism by which Pepcid mediates antiinflammatory activities recorded both clinically and in models of inflammatory diseases in animals. The antiinflammatory effects generated by Pepcid depend on alpha 7 nicotinic acetylcholine receptors (Yang H,et al. Famotidine activates the vagus nerve inflammatory reflex to attenuate cytokine storm. Mol Med. 2022; 28(1):57. doi: 10.1186/s10020-022-00483-8), the same receptor system utilized by acetylcholine to mediate antiinflammatory effects by the vagus system.
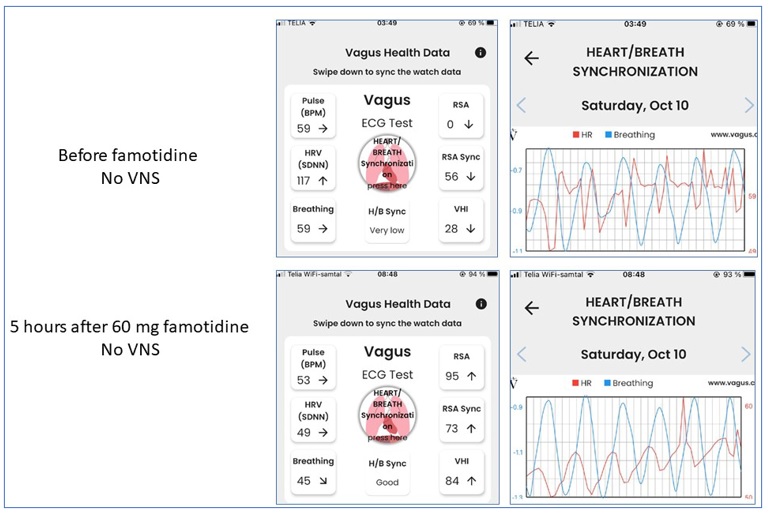
Fig 5. Oral intake of 60 mg famotidine restored the poor heart/breath synchronization without any need for taVNS.
Based on the facts that both taVNS and Pepcid have antiinflammatory effects and generate the same changes of the patient’s Vagus ECG Smartwatch recordings, we hypothesize the following: the patient’s poor synchronization of HRV and breathing pattern reflects abnormal vagus signaling that includes reduced cholinergic antiinflammatory capacity. The observation that the synchronization is normalised after taVNS suggests that normalised cholinergic antiinflammatory capacity can be re-established by VNS. Further studies of synchronization in patients with chronic inflammatory diseases that respond to any form of VNS should be able to shed further light on this hypothesis. Animal HRV studies have demonstrated that activation of nicotinic acetylcholine receptors regulates the synchronization of heart rate changes during respiration (Neff RA, et al.). The issue of synchronization has, to our knowledge not been much discussed in the context of vagal tone measurements.
DISCUSSION
Pathogenesis of the chronic inflammation
We want to discuss aspects of the pathogenesis of the systemic inflammation and neuroinflammation in the perspective of HMGB1 and the beneficial effects mediated by taVNS and acetylcholine. Most gut bacteria are Gram-negative bacteria, which all express LPS in the outer cell membrane. Purified LPS is the golden standard molecule to induce inflammation and production of inflammatory mediators in animal experiments and in cell cultures. Acute life-threatening systemic inflammation will follow when LPS is injected in high doses in animals, while providing repeated low doses over long time is an established method to cause experimental depression. Most of the gut bacteria that continuously enter the bile tract are thus Gram-negative bacteria releasing LPS that will be taken up in circulation to reach and damage the blood-brain barrier to induce neuroinflammation in the central nervous system. Depending on the site of the inflammation in the brain, almost any symptom may emerge. For the patient, depression, anxiety, and cognitive decline have been the dominating consequences of the neuroinflammation. LPS on its own is a potent proinflammatory molecule, but it also readily forms a complex with the endogenous human protein HMGB1 and then creates an even much more dangerous molecule. HMGB1 is continuously released in the liver due to the ongoing inflammation in chronic cholangitis and in large quantities during acute episodes of cholangitis. HMGB1 is present in all nucleated cells, but the hepatocytes in the liver are the cells that express the most abundant quantities of HMGB1. Acute flare-ups of liver inflammation will thus generate huge amounts of extracellular HMGB1-LPS complexes that will reach the brain and cause damage and inflammation there. Most proinflammatory mediators mediate their effects by binding and signaling via specific cell surface receptors expressed on target cells. Intracellular signals will then be transmitted to the nucleus inducing transcription for factors executing the functions intended by the extracellular mediator. When LPS acts on its own it binds and signals via such a surface-expressed receptor (TLR4; the identification of this molecule was associated with the 2011 Nobel Prize in Physiology or Medicine). When LPS is attached to HMGB1 the complex is endocytosed via a cell surface receptor named RAGE into the cytoplasm of the target cell where two intracellular LPS-receptors (caspases 4 and 5) are located. These intracellular receptors are much more potent than the cell surface receptor (TLR4) and the inflammation will be much stronger. Without the intracellular HMGB1-assisted transport, LPS would never reach its dangerous cognate intracellular receptor. To make a long story shorter, the chronic bile tract infection with acute exacerbations generates complexes of LPS and extracellular HMGB1 which cause neuroinflammation and systemic inflammation causing the symptoms.
How did taVNS ameliorate the inflammation?
What about acetylcholine and vagus nerve stimulation in this context? Clinical and preclinical observations have confirmed the existence of the cholinergic antiinflammatory system mediating its effects via acetylcholine release from the vagus nerve acting on target cells via alpha 7 nicotinic acetylcholine receptors (a7nAhR) to inhibit the release of proinflammatory mediators. It has also been established that electrical stimulation using defined settings can restore a cholinergic antiinflammatory system expressing reduced functionality. A fascinating fact is that both acetylcholine and HMGB1, which are two evolutionarily very ancient and conserved molecules, act as yin and yang in the neuronal control of inflammation. When acetylcholine binds to a7nAChR on the surface of a target cell the interaction results in activation of specific enzymes (histone deacetylases) in the cellular nucleus and these enzymes remove acetyl groups from nuclear proteins (Li DJ, et al.). The nuclear protein HMGB1 needs to express acetyl groups to be authorized to escape the nucleus, otherwise HMGB1 will remain in the nucleus and cannot be released extracellularly to promote inflammation. When taVNS enhances the release of acetylcholine, this can reduce the extracellular amounts of the two pathogenic molecules, which are extracellular HMGB1 and HMGB1-LPS complexes.
CONCLUSIONS
We here speculate on explanations for why taVNS mediated such strong beneficial effects as a treatment for the patient´s depression and generalized anxiety, symptoms generated by inflammation in the central nervous system. The neuroinflammation was secondary to a persistent abdominal Gram-negative bacterial infection in the bile tract and pancreas. This chronic infection was an inevitable consequence after extensive abdominal surgery performed 20 years earlier leaving the bile tract and pancreatic ducts persistently accessible for an influx of intestinal bacteria instead of remaining sterile. One key molecule driving the chronic systemic inflammation was the proinflammatory mediator LPS released from the Gram-negative bacteria translocated from the gut to the liver and pancreas. The potent proinflammatory endogenous molecule HMGB1 released from stressed liver and pancreas cells conceivably aggravated the toxicity of LPS by forming complexes that passed the blood-brain barrier and caused neuroinflammation. Acetylcholine discharged after taVNS prevented extracellular release of HMGB1 and thus ameliorated inflammation. Acetylcholine activates a7 nicotinic acetylcholine receptors to stimulate nuclear histone deacetylase activity, which prevents HMGB1 from escaping the nucleus to reach the cytoplasm. When HMGB1 is retained in the nucleus the molecule cannot reach the extracellular environment to induce inflammation.
Results from the Vagus ECG Smartwatch recordings suggest exciting clues to monitor and optimize taVNS therapy. The patient´s synchronization (RSA and RSA Sync) of heart rate changes during controlled breathing was constantly very poor but normalized each time after taVNS or intake of the antiinflammatory compound famotidine (Pepcid). Famotidine acts via alpha 7 nicotinic acetylcholine receptors to inhibit inflammation. The vagus system regulates the synchronization as well as the cholinergic antiinflammatory mechanism via nicotinergic acetylcholine receptors. We thus hypothesize that RSA and RSA Sync recordings might be used to monitor and evaluate vagus nerve signaling required for the regulation of the cholinergic antiinflammatory mechanism. In contrast, heart rate variability measured as HRV (SDNN) did not change in any consistent direction after taVNS and did thus not indicate improved vagus tonicity as a consequence of the neuronal stimulation. This result was not totally unexpected since HRV (SDNN) recordings reflect both parasympathetic and sympathetic signaling.
References
Li DJ, et al. α7 Nicotinic Acetylcholine Receptor Relieves Angiotensin II-Induced Senescence in Vascular Smooth Muscle Cells by Raising Nicotinamide Adenine Dinucleotide-Dependent SIRT1 Activity. Arterioscler Thromb Vasc Biol. 2016; 36(8):1566-76. doi: 10.1161/ATVBAHA.116.307157 Neff RA, et al. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003; 93(6):565-72. doi: 10.1161/01.R