We are open to partnering, contact our team at
Phase 2 Ready Orally Administered p38 MAP Kinase inhibitor
Severe influenza is typically characterised by a disproportionate systemic immune response, which can cause significant local damage in the lungs and can affect other organs in the body. The uncontrolled immune response commonly results in a need for hospitalization.
POLB 001 selectively inhibits overwhelming inflammation in severe influenza, while leaving the necessary immune functions intact to fight the infection. This contrasts with other immunomodulatory approaches, such as steroids, which affect both beneficial and damaging immune responses.

Compelling Data
- Phase 2 ready oral small molecule, excellent bioavailability
- Strong pre-clinical data package
- Proven safety profile & well tolerated in Phase I clinical trial
- Efficacy demonstrated in Phase 1b human challenge trial supports partnering
Strong Patent Portfolio
- Granted patents for severe influenza out to 2038
- Oncology patent applications filed in 2023, potential for protection out to 2043
- Learn more about POLB 001 for cancer immunotherapy-induced CRS here
Major Market Opportunity
- 1 Billion estimated cases of influenza each year, 5-10 million hospitalisations, 500,000 annual influenza related deaths
- Current treatments are effective within the first 48 hours of symptom onset but are less effective at more advanced stages of the disease
- POLB 001 is strain agnostic and shelf stable making it ideal for stockpiling
Evidence for benefit of POLB 001 in the therapy of LPS-induced inflammation
Supplementing the applicability of POLB 001 to severe influenza on the one hand and immunotherapy-induced CRS on the other, POLB 001 has been investigated in a human lipopolysaccharide (LPS) challenge trial.
Randomised, double-blind, placebo-controlled, multiple dose, inflammatory challenge trial in healthy volunteers
Trial design

Endpoints
Intravenous LPS challenge
- Bloods (cytokines, vascular markers, CRP)
- Ex-vivo LPS response
- Safety & tolerability (inc. vital signs, AE’s, ECG, Haematology)
- Local inflammatory responses were also measured
Results Demonstrate POLB 001 can Potently Inhibit Inflammation
POLB 001 was widely distributed, reduced the inflammatory response and inhibited p38 MAPK activation and signaling following LPS challenge.
- Excellent safety profile across two clinical studies
- Potent target inhibition confirmed
- Major reduction of key inflammatory markers
- Clear dose response relationship observed
Potent and Selective Inhibition of p38 MAPK Signaling
Effective target engagement demonstrated

Levels of phosphorylated p38 MAPK in circulating monocytes
- POLB 001 was widely distributed
- POLB 001 inhibited p38 MAPK activation, direct measurement of activation
- POLB 001 inhibited in vivo and ex vivo responses to LPS-induced TNF-α, indirect measurement p38 activity
Blood samples were taken before and after administration of intravenous LPS. Peripheral blood samples were analyzed by flow cytometry. Monocytes were gated by FSC, SSC and CD14+. Data is presented as mean MFI values of phospho-p38 +/- SEM
Reduced Key Inflammatory Cytokines Following LPS Challenge
Dose dependent reductions of inflammation
TNF-α

TNF-α reduction of 73.5% and 56.2% seen for 70 mg and 150 mg doses respectively (p = 0.0003†)
IL-6

IL-6 reduction of 57.4% and 63.5% seen for 70 mg and 150 mg doses respectively (p = 0.0002†)
IL-8

IL-8 reduction of 80.7% and 76.7% seen for 70 mg and 150 mg doses respectively (p < 0.0001†)

TNF-α, IL-6 and IL-8 levels decreased between 56-81% in subjects treated with 70 mg or 150 mg POLB 001 twice daily
†The exploratory analysis suggested statistically significant improvement in treatment (p<0.05) for the endpoints examined.
Reduced Key Indicators of LPS-Induced Systemic Inflammation
The reduction of systemic cytokines aligns with improvement in clinically meaningful endpoints
Mean Body Temperature

No significant effect on body temperature with a trend towards reduction compared to placebo.
Heart Rate Rise (bpm)

Suppressed increase in heart rate following IV LPS administration
C-Reactive Protein (CRP)

CRP level reductionof 33.1% and 33.3% seen for 70mg and 150mg doses respectively

POLB 001 Effectively Reduced Inflammation in Tissue
POLB 001 150 mg significantly reduced IL-1β† and TNF-α† responses in blister exudate compared to placebo
TNF-α in blister exudate
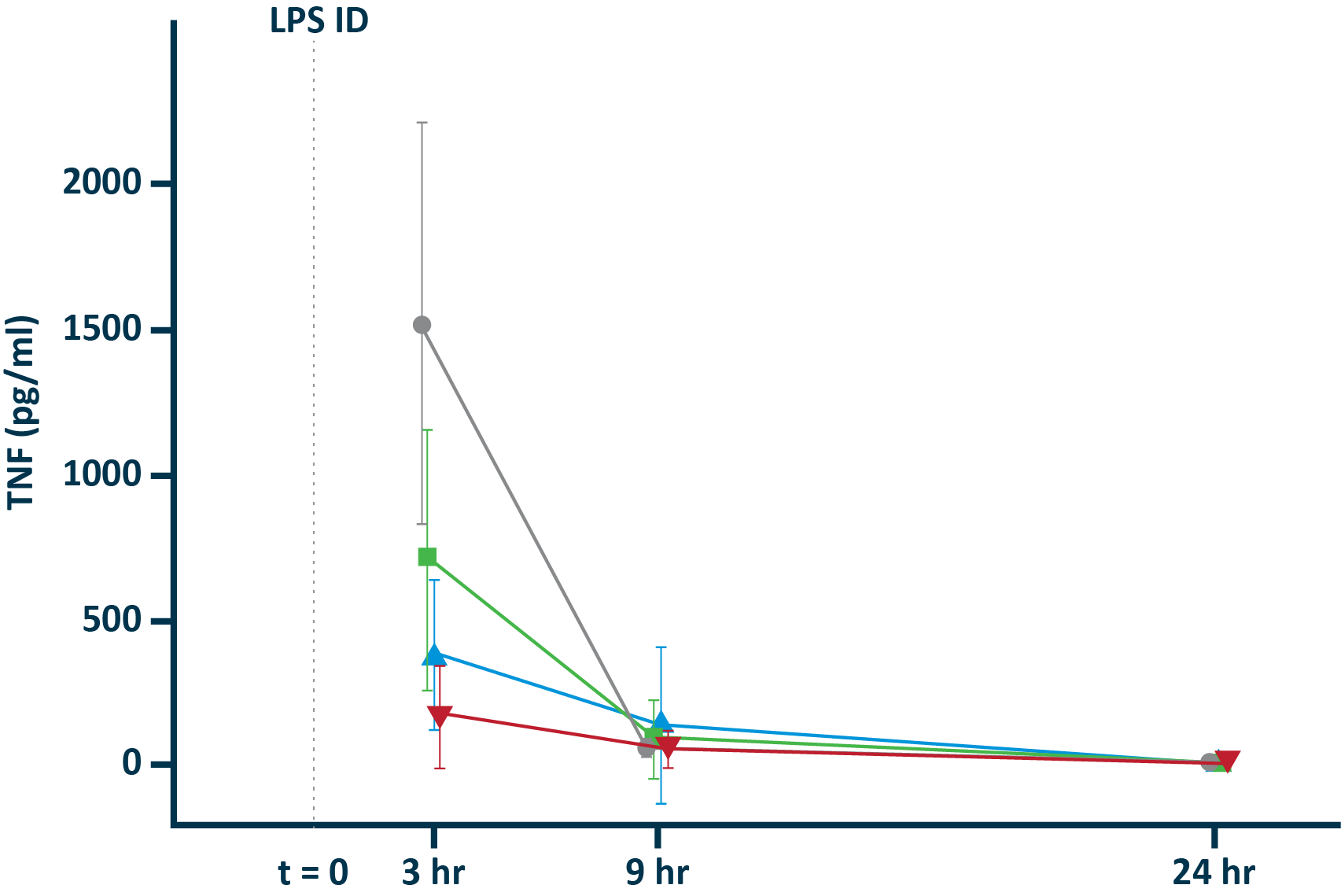
IL-1β in blister exudate
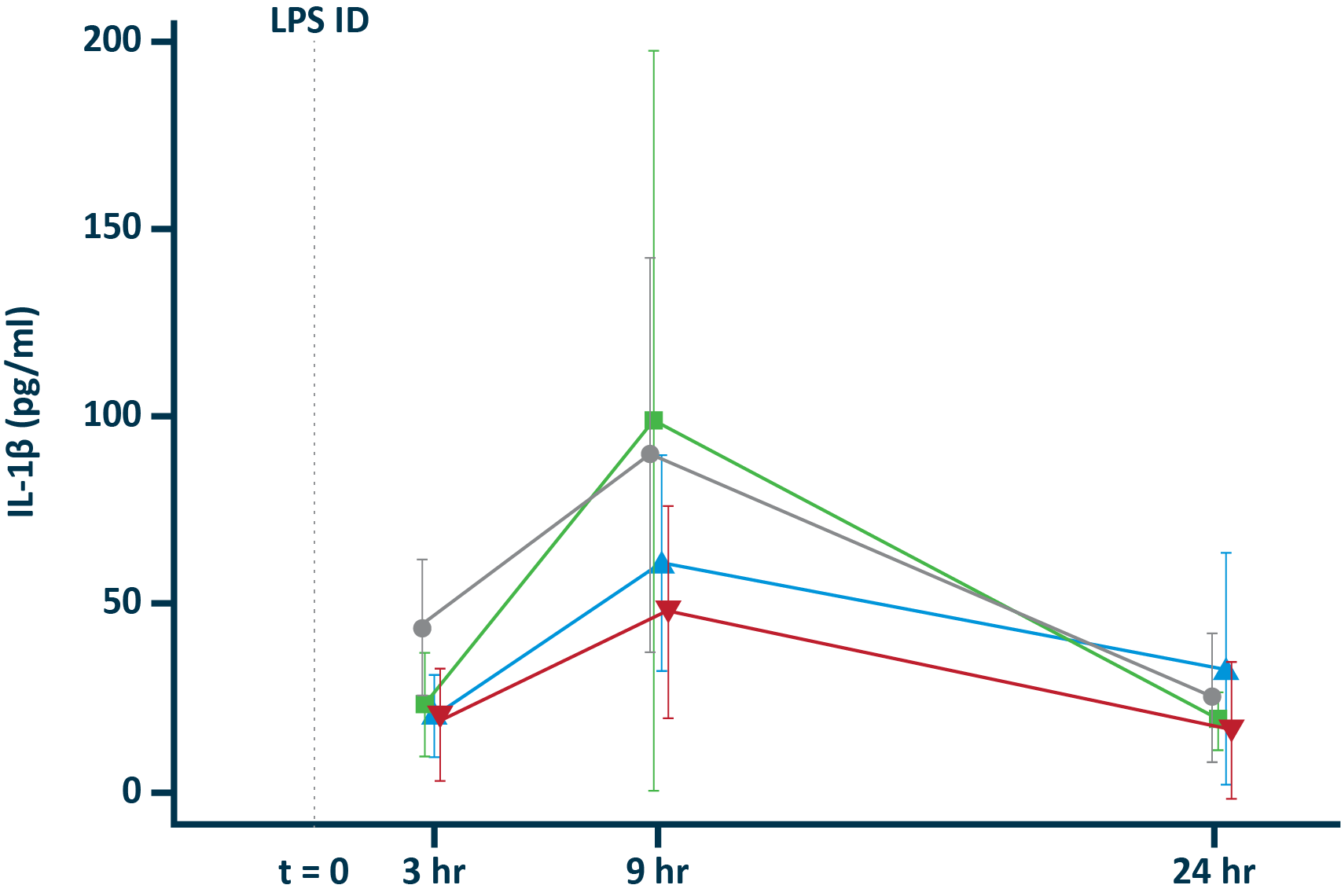
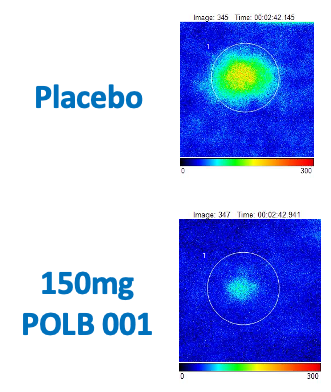

†The exploratory analysis suggested statistically significant improvement in treatment (p<0.05) for the endpoints examined.






