Abstract
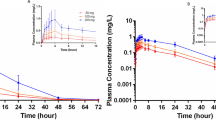
The pharmacokinetics of colchicine were studied in six healthy male and four elderly female volunteers after i. v. and oral administration. Plasma samples were collected over 72 h and assayed for colchicine by a specific and sensitive radioimmunoassay. Plasma concentration-time curves were fitted using a three-compartmental model after i. v. administration of 0.5 mg (healthy volunteers) and 1 mg (elderly group) colchicine. The first distribution half-life (t1/2 λ1) was short: 9.2 min in healthy volunteers and 3.0 min in the elderly group; the second distribution half-life (t1/2 λ2) was of the same order for both groups, 1.2 h. Plasma elimination half-lives were also in the same range: 30 h for healthy volunteers versus 34 h for the elderly subjects. Mean residence time was also in the same range in the two groups: 27 h in healthy volunteers and 21 h for elderly subjects. The volume of distribution (Vz) was 6.71·kg-1 for the healthy group and 6.31·kg-1 for the elderly group, while Vss was smaller: 4.21·kg-1 for healthy volunteers and 2.91·kg-1 for elderly subjects. Total body clearance was 10.51·h-1 for healthy and 5.51·h-1 for elderly subjects.
After oral administration of 1 mg, lag-time was 14 min in healthy volunteers and 11 min in elderly subjects. Maximal plasma concentration was 5.5 ng·ml-1 at 62 min in the healthy group, while in the elderly group Cmax was 12 ng·ml-1 at 87 min. Mean absolute bioavailability of the tablet was the same in both groups, 44% for healthy volunteers and 45% for elderly subjects.
Similar content being viewed by others
References
Roberts W, Liang M, Stern S (1987) Colchicine in acute gout. Am Med Ass 257: 1920–1922
Levy M, Eliakim M (1977) Long-term colchicine prophylaxis in familial Mediterranean fever. BMJ 2: 808–809
Tafi L, Matucci-Cerinic M, Falcini F, Valentini G, Bartolozzi G (1973) Colchicine treatment of Behcet's disease in children. Arthritis Rheum 30: 1435
Alarcon-Segovia D, Ramos-Niembro F, de Kasep GI, Alcacer J, Perez Tamayo R (1979) Long-term evaluation of colchicine in the treatment of scleroderma. J Rheumatol 6: 705–712
Takigawa M, Miyachi Y, Uehara M, Tagami H (1982) Treatment of postulosis palmaris and plantaris with oral doses of colchicine. Arch Dermatol 118: 458–460
Kershennobich D, Vargas F, Garcia-Tsao G, Tamayo RP, Gent M, Rojkind M (1988) Colchicine in the treatment of cirrhosis of the liver. N Engl J Med 318: 1709–1713
Caplan YH, Orloff KG, Thompson BC (1980) A fatal overdose with colchicine. J Anal Toxicol 4: 153–155
Ertel NH, Mittler JC, Akgun S, Wallace SL (1976) Radioimmunoassay for colchicine in plasma and urine. Science 193: 233–235
Halkin H, Dany S, Greenwald M (1980) Colchicine kinetics in patients with familial Mediterranean fever. Clin Pharmacol Ther 28: 82–87
Galliot M, Bourdon R (1979) Colchicine: cinétique d'élimination au cours des traitements et des intoxications aiguës. 1 er Congrés hispano-français de Biopharmacie et Pharmacocinétique, Barcelona, 2–6 April
Scherrmann JM, Boudet L, Pontikis R, Nguyen-Hoang-Nam, Fournier PE (1980) A sensitive radioimmunoassay for colchicine. J Pharm Pharmacol 32: 800–802
Thomas G, Girre C, Scherrmann JM, Francheteau P, Steimer JL (1989) Zero-order absorption and linear disposition of oral colchicine in healthy volunteers. Eur J Clin Pharmacol 37: 79–84
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Dekker, New York
Yamoaka K, Nakagawa T, Uno T (1978) Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 6: 165–175
Jarvie D, Park J, Stewart MJ (1979) Estimation of colchicine in a poisoned patient by using high performance liquid chromatography. Clin Toxicol 14: 375–381
Girre C, Thomas G, Scherrmann JM, Crouzette J, Fournier PE (1989) Model-independent pharmacokinetics of colchicine after oral administration to healthy volunteers. Fundam Clin Pharmacol 3: 537–543
Wagner JG (1974) Application of the Wagner-Nelson absorption method to the two-compartment open model. J Pharmacokinet Biopharm 2: 469–486
Achtert G, Scherrmann JM, Christen MO (1989) Pharmacokinetics/Bioavailability of colchicine in healthy male volunteers. Eur J Drug Metab Pharmacokinet 14: 317–322
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rochdi, M., Sabouraud, A., Girre, C. et al. Pharmacokinetics and absolute bioavailability of colchicine after i. v. and oral administration in healthy human volunteers and elderly subjects. Eur J Clin Pharmacol 46, 351–354 (1994). https://doi.org/10.1007/BF00194404
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00194404