Pioneering Regenerative Medicine
Without Immunosuppression
We are a data-driven and patient-centric regenerative medicine company working to enable implantable tissue, organoid or cell therapy without the requirement for life-long immunosuppression. Leveraging our proprietary technology platform, we are advancing a pipeline of product candidates that have the potential to cure diseases.
Pioneering Regenerative Medicine
Without Immunosuppression

We are a data-driven and patient-centric regenerative medicine company working to enable implantable tissue, organoid or cell therapy without the requirement for life-long immunosuppression. Leveraging our proprietary technology platform, we are advancing a pipeline of product candidates that have the potential to cure diseases.
iTOL-100:
Biotechnology-Derived Protein Inducing
Immune Tolerance Platform
iTOL-100 is a synthetic form of the naturally occurring protein, FasL, combined with a synthetic biotin-PEG microgel (SA-FasL biotin-PEG microgel) that allows convenient and effective co-administration with implanted cells or organoids to induce local immune tolerance without the need for life-long immunosuppression.
iTOL-100:
Biotechnology-Derived Protein Inducing
Immune Tolerance Platform
iTOL-100 is a synthetic form of the naturally occurring protein, FasL, combined with a synthetic biotin-PEG microgel (SA-FasL biotin-PEG microgel) that allows convenient and effective co-administration with implanted cells or organoids to induce local immune tolerance without the need for life-long immunosuppression.

Developing a Potential Cure for Type 1 Diabetes
Our first in human study, iTOL-101 is planned as a proof-of-concept study utilizing donor-derived allogeneic pancreatic islets while our lead commercial program is iTOL-102; both a potential cure for Type 1 Diabetes. iTOL-102 utilizes transplanted human stem-cell derived pancreatic islets combined with iTolerance’s proprietary platform, iTOL-100, which induces local immune tolerance thus eliminating the need for life-long chronic immunosuppression. In a pre-clinical non-human primate studyallogeneic pancreatic islet cells co-implanted with SA-FasL biotin-PEG microgel (iTOL-100) exhibited long-term (6 months) function with control of blood glucose levels and restoration of insulin secretion without the use of chronic immune suppression. iTOL-102 is leveraging significant advancements in stem cells to derive pancreatic islets which allows an inexhaustible supply of insulin-producing cells for use in Type 1 Diabetes.
Development Pipeline
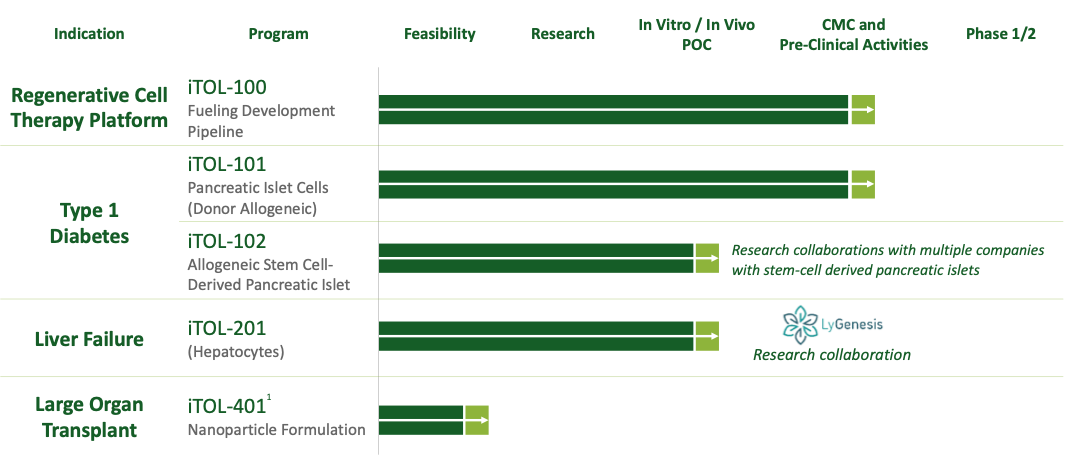
1: Development of iTOL-401 is subject to the entry of a license agreement with Georgia Tech Research Corporation
We Were 1 of 3 Companies Selected to Participate in the
Disruptive Healthcare Technology Showcase at the US Capitol

Email Alerts:
Have the latest news and information delivered directly to your inbox
Get in Touch with us:
Please reach out with any questions
News:
Email Alerts:
Have the latest news and information delivered directly to your inbox
Get in Touch with us:
Please reach out with any questions
News:
Kadimastem and iTolerance Complete Initial Targeted Engagement for Regulatory Advice on CBER Products (INTERACT) Meeting with the U.S. FDA
Companies Advancing Joint Clinical Development of a Potential Cure for Diabetes Without the Need for Chronic Suppression of the Immune System Ness Ziona, Israel, January 22, 2024, Kadimastem (TASE: KDST) and iTolerance Inc. announced today that the companies held a...
iTolerance, Inc. to Present at Biotech Showcase™ 2024
Live presentation on Monday, January 8th at 11:30 AM PTMIAMI, FL / January 4, 2024 / iTolerance, Inc. ("iTolerance" or the "Company"), an early-stage, privately-held biotechnology company focused on the development of innovative regenerative medicines, today announced...
iTolerance, Inc. to Present at Biotech Showcase(TM) 2024
Live presentation on Monday, January 8th at 11:30 AM PT MIAMI, FL / January 4, 2024 / iTolerance, Inc. ("iTolerance" or the "Company"), an early-stage, privately-held biotechnology company focused on the development of innovative regenerative medicines, today...


