Study overview
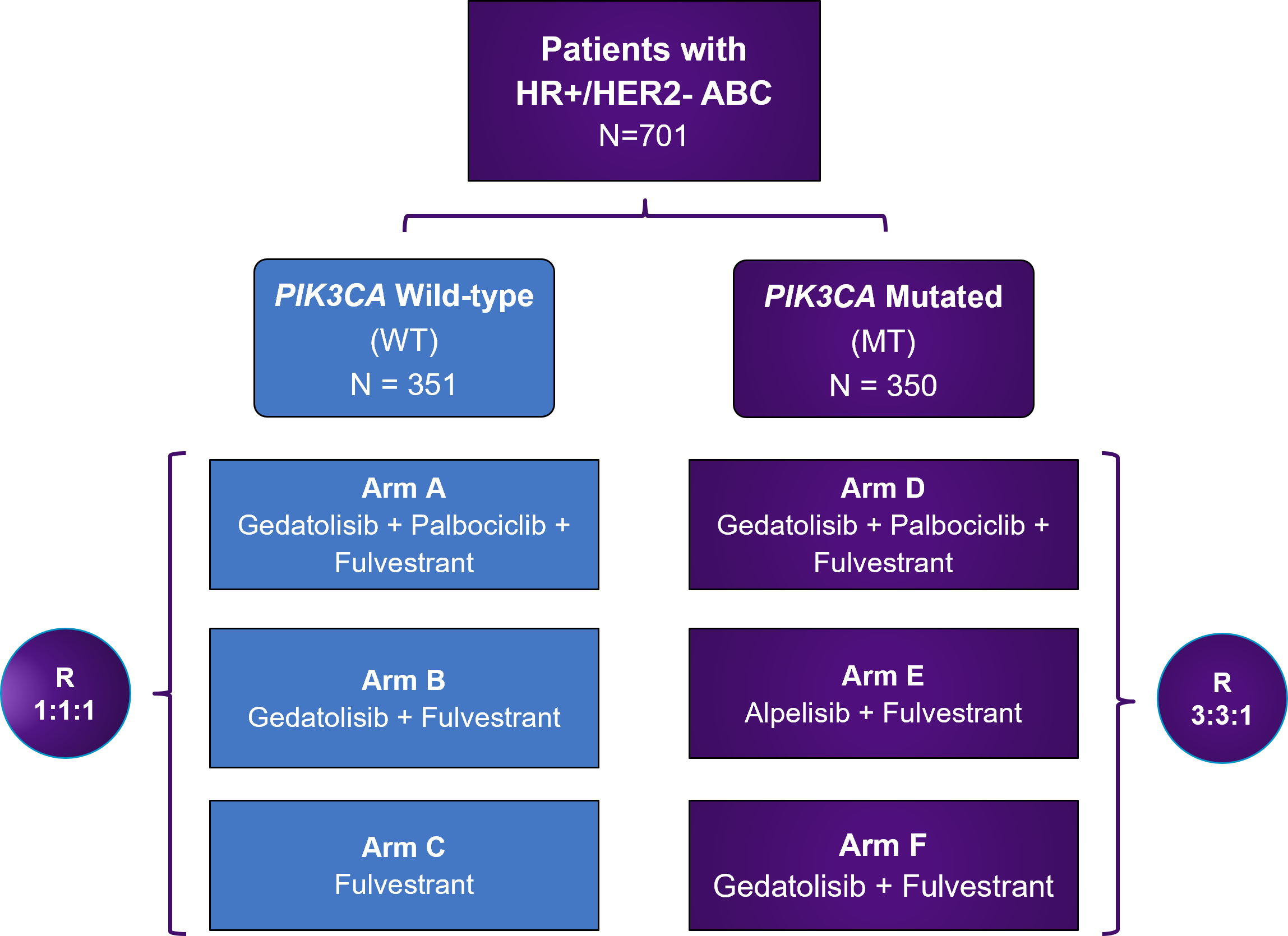
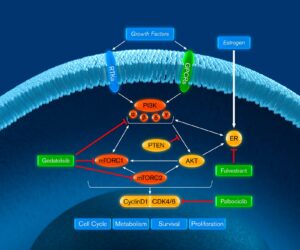
VIKTORIA-1 is a Phase 3 study evaluating gedatolisib plus fulvestrant with and without palbociclib in patients previously treated with a CDK4/6 therapy and an aromatase inhibitor.
Approximately 701 eligible patients whose PIK3CA mutational status has been determined will be enrolled.
Eligible patients who do not have confirmed PI3KCA mutations (WT) will be randomly assigned (1:1:1) to receive a regimen of either gedatolisib, palbociclib, and fulvestrant (Arm A), gedatolisib and fulvestrant (Arm B), or fulvestrant (Arm C).
Eligible patients who have confirmed PI3KCA mutations (MT) will be randomly assigned (3:3:1) to receive a regimen of either gedatolisib, palbociclib, and fulvestrant (Arm D), alpelisib and fulvestrant (Arm E) or gedatolisib and fulvestrant (Arm F).