Lipella Pharmaceuticals is a clinical-stage biotechnology company developing and commercializing treatments for serious diseases. Our proprietary drug delivery technology has potential applications in addressing diseases of the mucosal tissue including the bladder, urethra, oral cavity, esophagus and colon. We see strong potential for our technology in partnerships and commercial licensing agreements.
Lipella maintains a sterile manufacturing facility in Pittsburgh, PA for the production of clinical supplies and research products.
Click the button above to view Lipella Corporate Presentation, April 2024

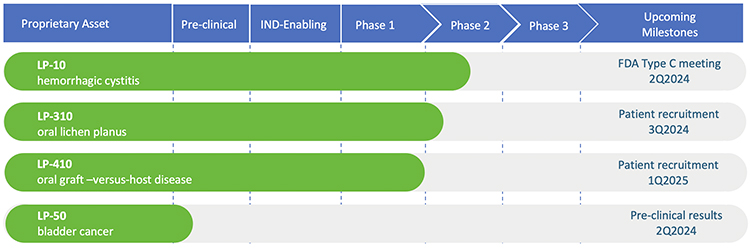
We recently completed a phase 2A multi-center dose-escalation clinical trial for our lead product, LP-10. Results are published in the peer-reviewed journal, International Urology and Nephrology. Our pipeline asset, LP-310, was granted approval to begin clinical trial by the FDA. We have received Orphan Drug Designation from the FDA for our drug candidate, LP-410, for the treatment of oral Graft-versus-Host Disease (GVHD). We maintain a pipeline of additional product candidates consistent with our strategy of developing proprietary 505(b)(2) assets that address highly-morbid indications where no adequate treatment(s) exists.
Radiation Cystitis Patient Registry
The following is a link to the Radiation Cystitis Patient Registry. If you are a cancer survivor with a history of pelvic radiation therapy, please consider joining the registry.
The potential uses of this registry about radiation cystitis and hemorrhagic cystitis (and cancer survivorship post-radiotherapy) include: improving the scientific understanding of radiation cystitis, discovering trends and common needs of registry participants, describing the aggregate personal characteristics of radiation cystitis patients within the registry, documenting registry patient medical histories, and contacting radiation cystitis registry participants to inform them of new studies. This registry has been created and developed within the published guidance of the National Institute of Health Rare Diseases Registry Program (RaDaR).




Reach Out