Phytochemical, Cytotoxicity, Antioxidant and Anti-Inflammatory Effects of Psilocybe Natalensis Magic Mushroom
Abstract
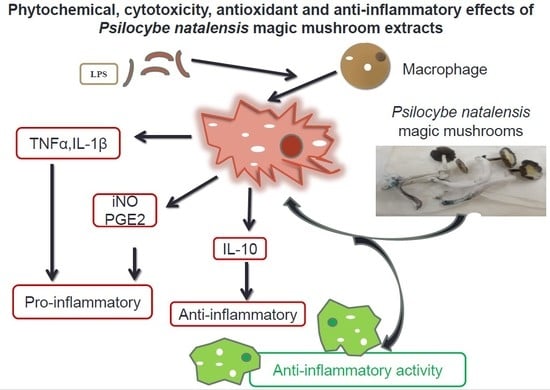
:1. Introduction
2. Results
2.1. ABTS Free Radical Scavenging Activity of P. natalensis Extracts
2.2. Cytotoxicity of P. natalensis Extracts
2.3. Anti-Inflammatory Effects of the Extracts
2.3.1. Inhibitory Effects of P. natalensis Extracts on Inducible NO Production and % Cell Viability
2.3.2. Effects of P. natalensis Extracts on PGE2 Production
2.3.3. Effects of P. natalensis Extracts on Cytokine Production
2.3.4. Phytochemical Determination
3. Discussion
4. Materials and Methods
4.1. Ethical Clearances
4.2. Mushroom Growth and Making Extracts
4.3. Free Radical Scavenging Activity on ABTS
4.4. Cytotoxicity of Extracts on Vero Normal Cells
4.5. Anti-Inflammatory Effects of the Extracts
4.5.1. RAW 264.7 Macrophage Cell Culture
4.5.2. Cytotoxicity of Extracts on LPS-Induced RAW 264.7 Macrophages
4.5.3. Treatment with the Extracts
4.5.4. Nitrite Content Measurements
4.5.5. PGE2 Activity Measurements
4.5.6. Cytokine Activity Measurements
4.6. Phytochemical Determination of the Extracts
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhong, J.; Shi, G. Editorial: Regulation of Inflammation in Chronic Disease. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, G.; Trusso, M.A.; Fagiolini, A. Depression and Inflammation: Disentangling a Clear Yet Complex and Multifaceted Link. Neuropsychiatry 2017, 7, 448–457. [Google Scholar] [CrossRef]
- Lucey, D.R.; Clerici, M.; Shearer, G.M. Type 1 and type 2 cytokine dysregulation in human infectious neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 1996, 9, 532–562. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.; Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012, 36, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Vuolteenaho, K.; Moilanen, T.; Knowles, R.G.; Moilanen, E. The role of nitric oxide in osteoarthritis. Scand. J. Rheumatol. 2007, 36, 247–258. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernández, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef]
- Johnson, M.W.; Griffiths, R.R. Potential Therapeutic Effects of Psilocybin. Neurotherapeutics 2017, 14, 734–740. [Google Scholar] [CrossRef]
- Guzman, G.; Allen, J.W.; Gartz, J. A worldwide geographical distribution of the neurotropica fungi, an analysis and discussion. Ann. Mus. Civ. Rovereto 1998, 14, 189–280. [Google Scholar]
- Van Amsterdam, J.G.; Opperhuizen, A.; Brink, W.V.D. Harm potential of magic mushroom use: A review. Regul. Toxicol. Pharmacol. 2011, 59, 423–429. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Alves, M.G.C.F.; Santos, M.G.L.; de Sauza, L.A.R.; Baseia, I.G.; Leite, E.L. Antioxiant and anti-inflammatory properties of an extract rich in polysaccharides of the mushroom Polyporus dermoporus. Antioxidants 2014, 3, 730–744. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-Inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Boil. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.; Kumaravel, S.; Latlitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res. 2010, 4, 191–195. [Google Scholar] [CrossRef]
- Palariya, D.; Singh, A.; Dhami, A.; Pant, A.K.; Kumar, R.; Prakash, O. Phytochemical analysis and screening of antioxidant, antibacterial and anti-inflammatory activity of essential oil of Premna mucronata Roxb. leaves. Trends Phytochem. Res. 2019, 3, 275–286. [Google Scholar]
- Maestre-Batlle, D.; Pena, O.M.; Huff, R.D.; Rhandhawa, A.; Carlisten, C.; Bolling, A.K. Dibutyl phthalate modulate phenotype of gradunulocytes in human blood in response to inflammation. Toxicol. Lett. 2018, 296, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodreza, M.; Younes, G.; Forough, K.; Hossein, T. Composition of the essential oil of Rosa damascene mill from South of Iran. Iran. J. Psychiatry Bahav. Sci. 2010, 6, 59–62. [Google Scholar]
- Ombito, O.J.; Matasyoh, C.J.; Vulule, M.J. Chemical composition and larvicidal activity of Zantoxylem gilletii essential oil against Anopheles gambie. Afr. J. Biotech. 2014, 13, 2175–2180. [Google Scholar] [CrossRef] [Green Version]
- Gurudeeban, S.; Sathyavani, K.; Ramanathan, T. Bitter apple (Citrulus colocynthis): An overview of chemical composition and biomedical potentials. Asian J. Plant Sci. 2010, 9, 394–401. [Google Scholar]
- Joo, T.; Sowndhararajan, K.; Hong, S.; Lee, J.; Park, S.-Y.; Kim, S.; Jhoo, J.-W. Inhibition of nitric oxide production in LPS-stimulated RAW 264.7 cells by stem bark of Ulmus pumila L. Saudi J. Boil. Sci. 2014, 21, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Phongpaichit, S.; Nikom, J.; Rungjindamai, N.; Sakayaroj, J.; Hutadilok-Towatana, N.; Rukachaisirikul, V.; Kirtikara, K. Biological activities of extracts from endophytic fungi isolated fromGarciniaplants. FEMS Immunol. Med. Microbiol. 2007, 51, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Mahavorasirikul, W.; Viyanant, V.; Chaijaroenkul, W.; Itharat, A.; Na-Bangchang, K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC Complement. Altern. Med. 2010, 10, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, D.; Yang, W.S.; Yang, Y.; Nam, G.; Kim, J.H.; Yoon, D.H.; Noh, H.J.; Lee, S.; Kim, T.W.; Sung, G.H.; et al. In vitro and in vivo anti-inflammatoty effect of Rhodomyrtus tomentosa methanol extract. J. Ethnopharmacol. 2014, 146, 205–213. [Google Scholar] [CrossRef] [PubMed]
- An, H.-J.; Kim, I.-T.; Park, H.-J.; Kim, H.-M.; Choi, J.-H.; Lee, K.-T. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2 and TNF-α expression through inactivation of the nuclear factor-κb pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2011, 11, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Tan, W.S.; Gothal, S.; Muniandy, K.; Fakurazi, S.; Esa, N.M.; Alarfaj, A.A.; Kumar, S.S. Anti-inflammatory potential of ethyl acetate fraction of Moringa oleifera in downregulating the NF-κb signalling pathways in lipopolysaccharide-stimulated macrophages. Molecules 2016, 21, 1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniadndy, K.; Gothai, S.; Badran, K.M.H.; Kumar, S.S.; Esa, N.M.; Arulselvan, P. Suppression of proinflammatory cytokine and mediators in LPS-induced RAW 264.7 macrophages by stem extract of Alternathera sessilis via the inhibition of the NF-κb pathway. J. Immunol. Res. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sanjeewa, K.K.A.; Nagahawatta, D.P.; Yang, H.-W.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; De Zoysa, M.; Whang, I.; Ryu, B. Octominin inhibits LPS-induced chemokine and pro-inflammatory cytokines secretion from RAW 264.7 macrophages via blocking TLRs/NF-κβ signal transduction. Biomolecules 2020, 10, 511. [Google Scholar] [CrossRef]
- Xie, Q.; Cho, H.; Calaycay, J.; Mumford, R.; Świderek, K.; Lee, T.; Ding, A.; Troso, T.; Nathan, C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992, 256, 225–228. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, J.; Brennan, C.; Ma, L.; Li, Y.; Lin, X.; Liu, H.; Wu, J. Anti-inflammatory effect of alkaloids extracted from Dendrobium aphyllum on macrophage RAW 264.7 cells through NO production and reduced IL-1, IL-6, TNF-α and PGE2 expression. Int. J. Food Sci. Technol. 2019, 55, 1255–1264. [Google Scholar] [CrossRef]
- Park, T.; Park, J.-S.; Sim, J.H.; Kim, S.-Y. 7-Acetoxycoumarin inhibits LPS-induced inflammatory cytokine synthesis by IκBα degradation and MAPK activation in macrophage cells. Molecules 2020, 25, 3124. [Google Scholar] [CrossRef]
- Kobelt, D.; Zhang, C.; Clayton-Lucey, I.A.; Glauben, R.; Voss, C.; Siegmund, B.; Stein, U. Pro-inflammatory TNF-α and IFN-γ Promote Tumor Growth and Metastasis via Induction of MACC1. Front. Immunol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Kuriakose, T.; Kanneganti, T.-D. Regulation and functions of NLRP3 inflammasome during influenza virus infection. Mol. Immunol. 2017, 86, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mwinga, J.L.; Asong, J.A.; Van Staden, J.; Nkadimeng, S.M.; McGaw, L.J.; Aremu, A.O.; Mbeng, W.O. In vitro antimicrobial effects of Hypoxis hemerocallidea against six pathogens with dermatological relevance and its phytochemical characterization and cytotoxicity evaluation. J. Ethnopharmacol. 2019, 242, 112048. [Google Scholar] [CrossRef] [PubMed]





| Sample | ABTS IC50 (μg/mL) | Vero LC50 (μg/mL) |
|---|---|---|
| Hot-water | 86.233 ± 2.370 | 49.080 ± 3.340 |
| Cold-water | 90.154 ± 8.748 | 25.046 ± 0.460 |
| 70% Ethanol | 26.586 ± 5.378 | 182.190 ± 11.860 |
| Ascorbic acid | 0.026 ± 0.003 | not applicable |
| Trolox | 0.928 ± 0.006 | not applicable |
| Doxorubicin | not applicable | 10.000 ± 1.327 |
| Compound Name | MW | Formula | Area % | Activity | Reference | ||
|---|---|---|---|---|---|---|---|
| Cold | Hot | Ethanol | |||||
| n-Hexadecanoic acid | 256 | C16H32O2 | 1.7129 | 2.0765 | 2.313 | Anti-inflammatory | [12] |
| Antioxidant | [13] | ||||||
| 4H-Pyran-4-one, 2,3-dihydro- | 144 | C6H8O4 | 2.0452 | Antioxidant | [13] | ||
| 3,5-dihydroxy-6-methyl- | Anti-inflammatory | ||||||
| 3-Octanone | 128 | C8H16O | 3.6742 | 3.2977 | Antioxidant | [14] | |
| Anti-inflammatory | |||||||
| Dibutyl phthalate | 278 | C16H22O4 | 12.383 | Anti-inflammatory | [15] | ||
| Nonadecane | 268 | C19H40 | 19.7244 | Antioxidant | [16,17] | ||
| Anti HIV | |||||||
| Antibacterial | |||||||
| Antimalarial | |||||||
| Tetradecane | 198 | C14H30 | 17.1872 | Anti-inflammatory | [18] | ||
| Antimicrobial | |||||||
| Anti-diarrhoeal | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkadimeng, S.M.; Nabatanzi, A.; Steinmann, C.M.L.; Eloff, J.N. Phytochemical, Cytotoxicity, Antioxidant and Anti-Inflammatory Effects of Psilocybe Natalensis Magic Mushroom. Plants 2020, 9, 1127. https://doi.org/10.3390/plants9091127
Nkadimeng SM, Nabatanzi A, Steinmann CML, Eloff JN. Phytochemical, Cytotoxicity, Antioxidant and Anti-Inflammatory Effects of Psilocybe Natalensis Magic Mushroom. Plants. 2020; 9(9):1127. https://doi.org/10.3390/plants9091127
Chicago/Turabian StyleNkadimeng, Sanah M., Alice Nabatanzi, Christiaan M.L. Steinmann, and Jacobus N. Eloff. 2020. "Phytochemical, Cytotoxicity, Antioxidant and Anti-Inflammatory Effects of Psilocybe Natalensis Magic Mushroom" Plants 9, no. 9: 1127. https://doi.org/10.3390/plants9091127
APA StyleNkadimeng, S. M., Nabatanzi, A., Steinmann, C. M. L., & Eloff, J. N. (2020). Phytochemical, Cytotoxicity, Antioxidant and Anti-Inflammatory Effects of Psilocybe Natalensis Magic Mushroom. Plants, 9(9), 1127. https://doi.org/10.3390/plants9091127