Initial Positive 6-Month Topline Efficacy Data From SAVVE Trial*
-
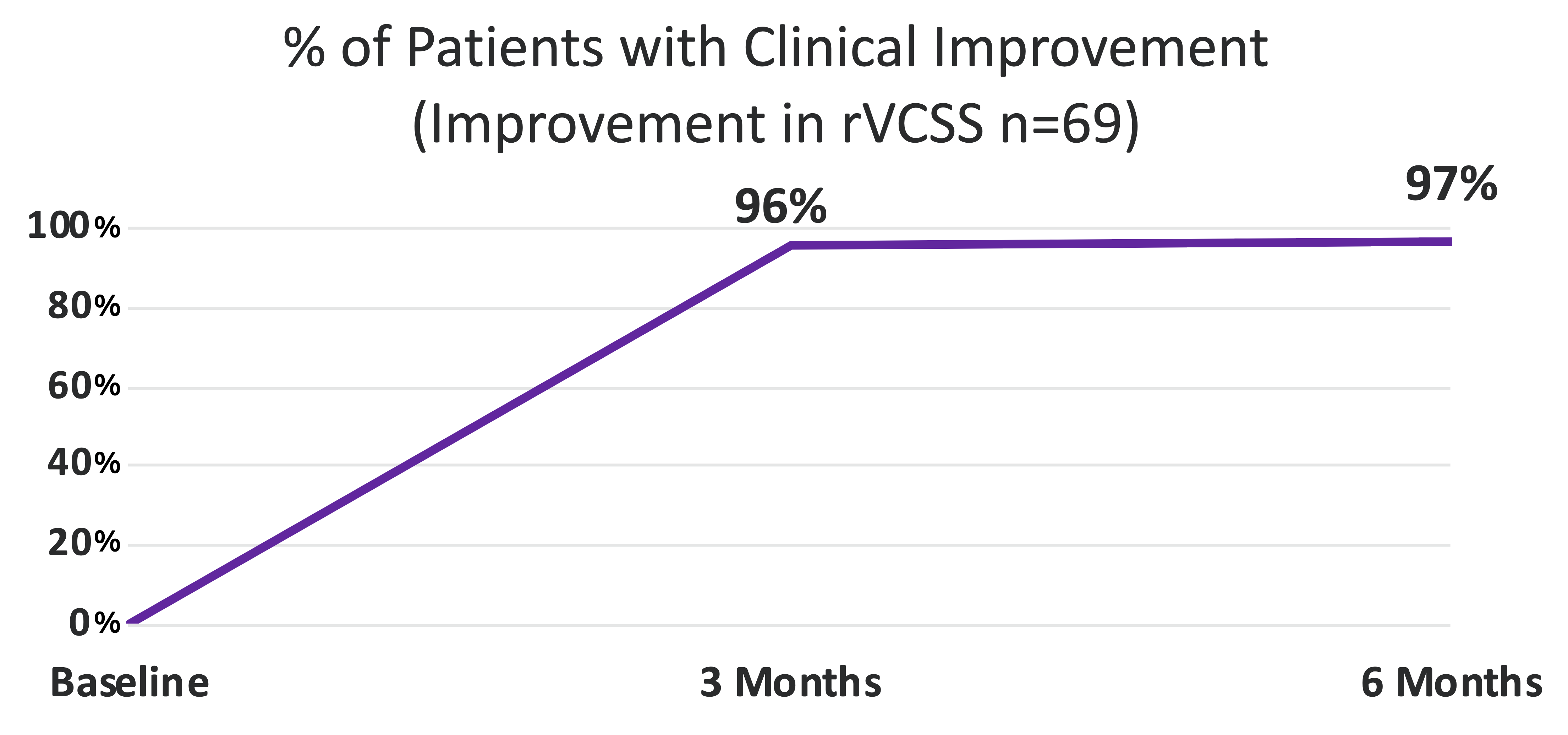
97% of all patients achieved Clinical Improvement (Improvement in rVCSS)
-
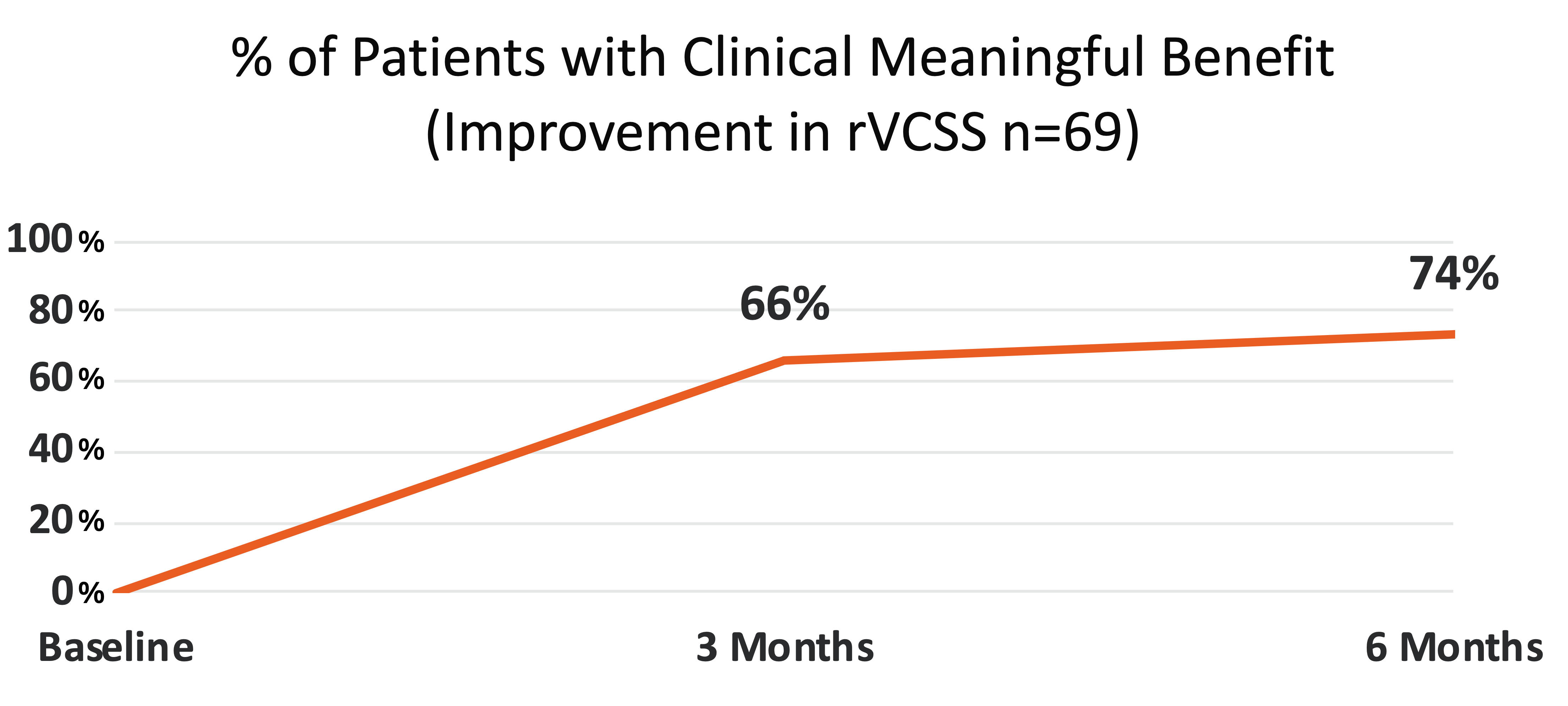
74% of all patients achieved a Clinical Meaningful Benefit (≥ 3 point improvement in rVCSS)
-
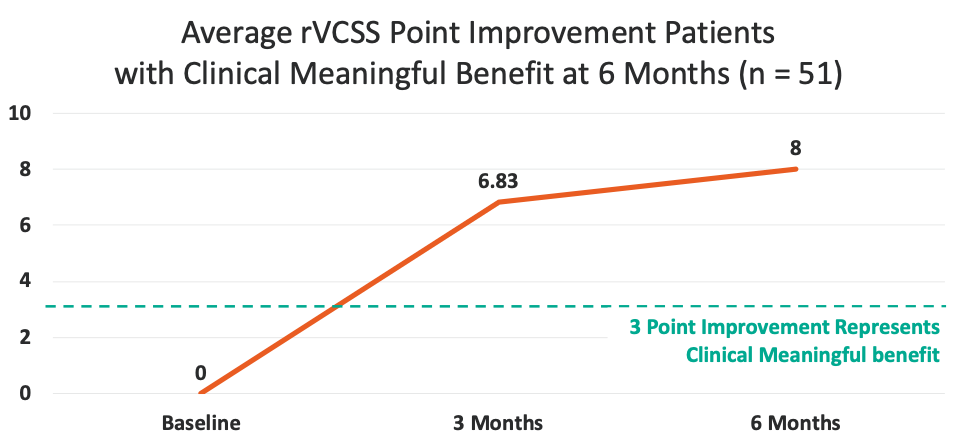
8 point average improvement in rVCSS
・More than two and a half times the amount needed to show Clinically Meaningful Benefit -
The revised Venous Clinical Severity Score (rVCSS) is an objective grading system used by vascular specialists throughout the world to measure the severity of venous diseases
* 6-month data compared to baseline
Setting New Standards for Venous Care
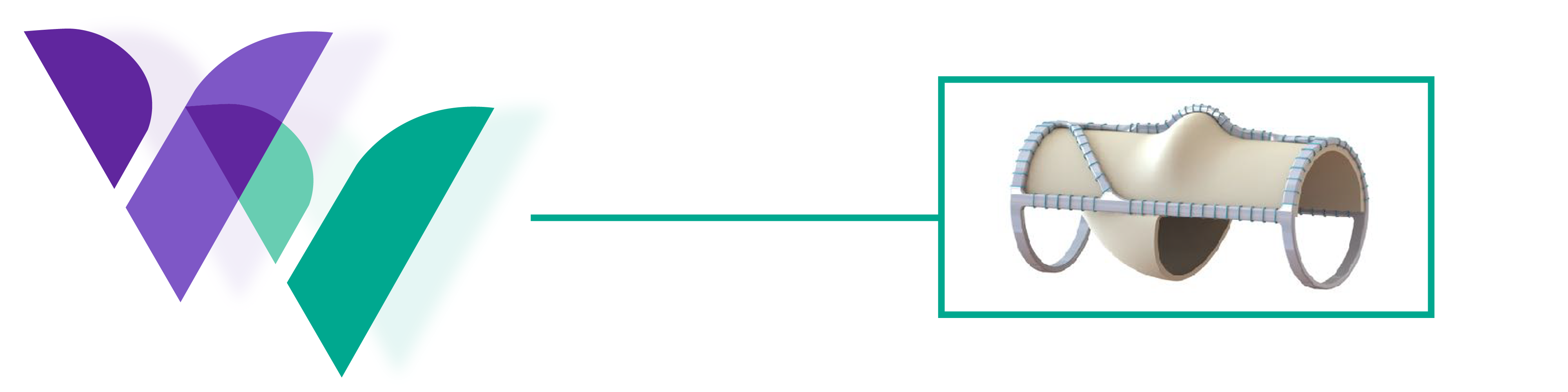
The VenoValve® is a first-in-class surgically implanted solution being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). Implanted into the femoral vein, the VenoValve is designed to act as a one-way valve to help restore proper blood flow up the leg, to return sufficient blood back to the heart. The VenoValve is currently being evaluated in the SAVVE® pivotal study.
Product
Highlights
-
FDA Breakthrough Device Designation
-
First device to receive IDE approval from the
FDA for the treatment of deep venous CVI
Product
Highlights
-
FDA Breakthrough Device Designation
-
First device to receive IDE approval from the
FDA for the treatment of deep venous CVI
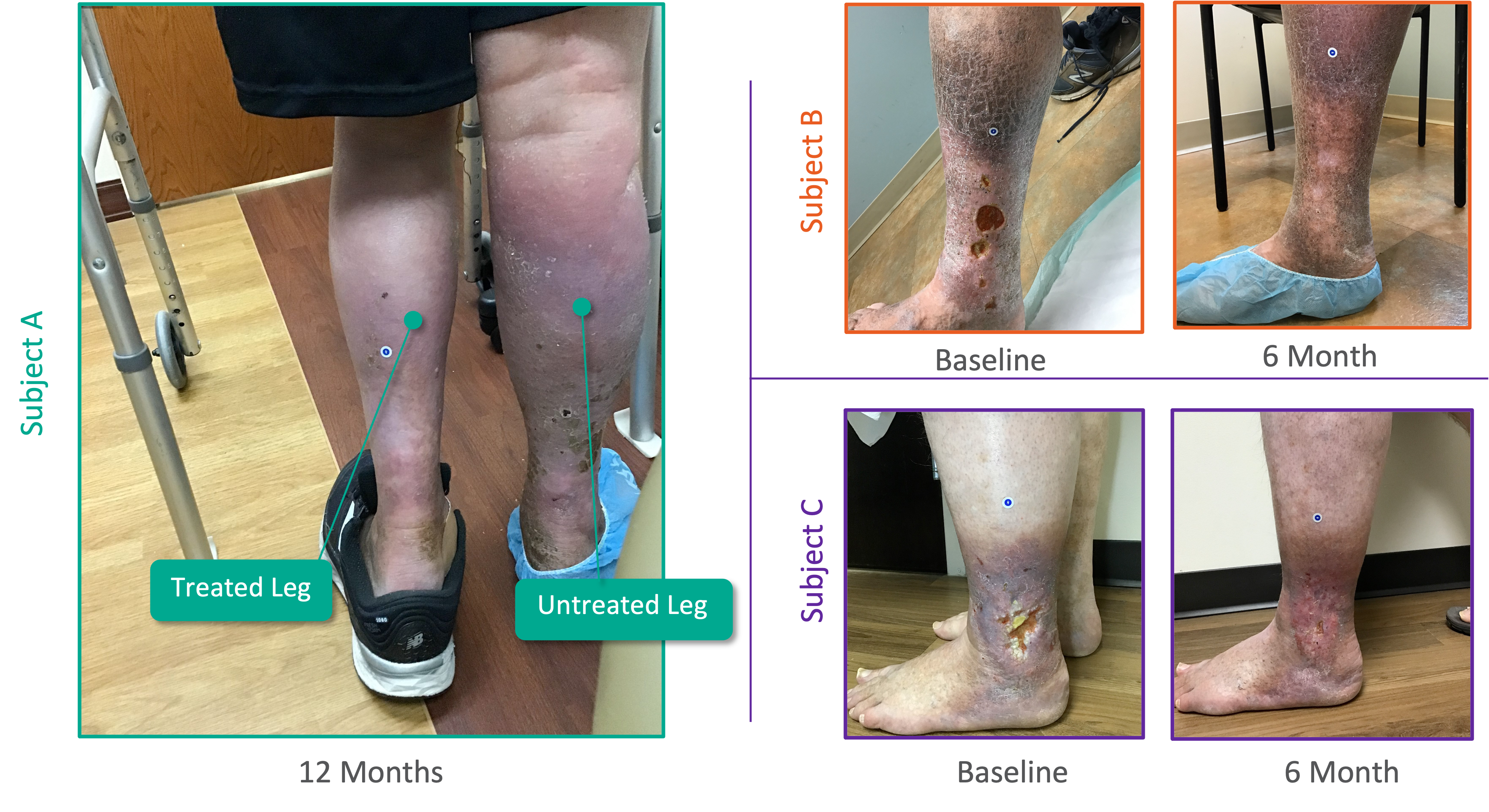
Patient Experience From Pivotal SAVVE® Study
Interviews from the 50th Annual VEITH Symposium
Initial Topline Results from SAVVE® U.S. Pivotal Trial
Topline Efficacy Data
Clinical Improvement
Improvement in rVCSS
- 97%
97% of VenoValve Study Patients Showing Clinical Improvement at Six Months Compared to Baseline (as Measured by the Revised Venous Clinical Severity Score (rVCSS)
Clinical Meaningful Benefit
≥ 3 Point Improvement in rVCSS
- 74%
74% of the Study Patients Showing Clinical Meaningful Benefit from the VenoValve at Six Months Compared to Baseline (Improvement in rVCSS of 3 or More Points)
Average rVCSS Improvement per Patient Showing Clinical Meaningful Benefit of 8 Points, More Than Two and a Half Times the Amount Needed to Show the VenoValve’s Clinically Meaningful Benefit
Compelling Device Related Safety Profile at 30 Days:*
0
No
Deaths
0
No Pulmonary
Embolisms
8%
Deep Vein Thrombosis
(DVT)**
*Higher than expected bleed rate was observed with no negative impact on clinical improvement
**Adjudication: 4 Mild; 2 Moderate. 5 of 6 DVT patients showing clinical improvement
Clinical Improvement = Improvement in rVCSS Compared to Baseline
Clinical Meaningful Benefit = Improvement in rVCSS ≥ 3 Points Compared to Baseline
8 Point Improvement Represents More than Two and a Half Times the Amount Needed to Show the VenoValve’s Clinically Meaningful Benefit
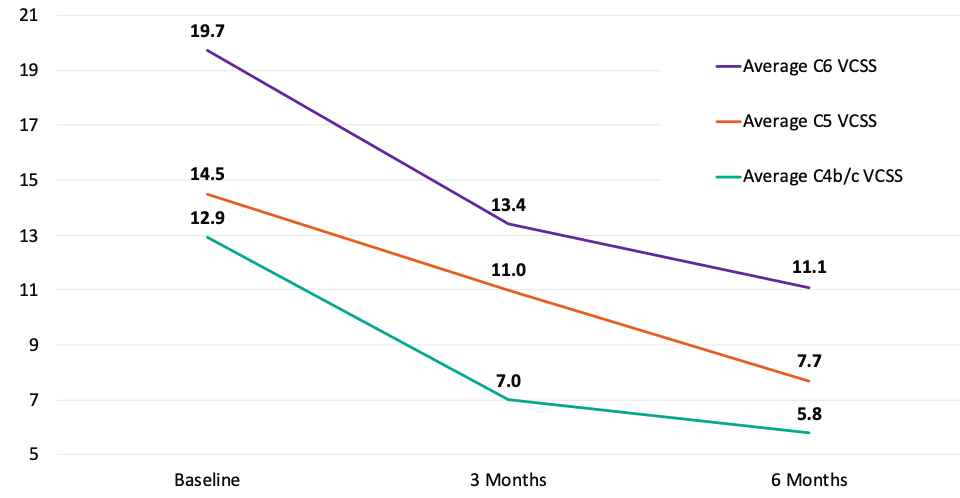
Average rVCSS Score by CEAP Diagnosis in Patients with Clinically Meaningful Benefit (N=51)
Patients with C4b/c, C5 and C6 are all benefiting from the VenoValve at 6 months compared to baseline.
Implantation Procedure
Chronic Venous Insufficiency
Scientific Publications
and Presentations
SAVVE® Clinical Study
The VenoValve® and enVVe® are investigational medical devices currently in development. Neither device is approved or cleared for any indication in any market. The VenoValve® is only available for use in the United States in pre-market clinical studies.