

Sometimes the most common things are overlooked. Although it is the eighth most abundant element in the universe and the seventh most common element in the earth’s crust, we seem to have a hard time getting the magnesium we need. It’s suspected that over 43% of Canadians don’t get the dietary magnesium they need to stay healthy.
That’s not good because it’s also supposed to be the fourth most abundant mineral in our bodies.
Elemental magnesium is found in high concentrations in our bones, our heart, our muscles and throughout our network of nerves, but you can also find it working away inside every cell of our bodies. Magnesium keeps heart rhythms steady, maintains muscle functions, metabolizes glucose, ensures nerves fire properly, creates cellular energy and helps to synthesize the basic building blocks of life – DNA, RNA and proteins. Over 800 different, essential biochemical roles that magnesium plays have been identified. With new research and understanding, that number is expected to rise.
Magnesium isn’t like a drug. While you might take an antacid to reduce heartburn or an antibiotic to fight infection, taking magnesium when you are deficient doesn’t have just one effect. Since magnesium works on such a fundamental level, the health benefits are hard to isolate. And because we can’t identify just one effect, it’s easy to overlook its importance.
But it is very important.
This mineral isn’t a drug we take only when the situation warrants. It’s more like a type of fuel or building block our body uses, just as our body uses sugars, proteins and fats. Vital organs like the heart and brain simply can’t function without it. Hundreds of your body’s chemical processes fall apart without this mineral. When we say our whole body needs magnesium to survive, we are not exaggerating.
We’re looking at magnesium the wrong way. There is a host of scientific making studies linking magnesium to many different health conditions, but reviewing these effects one by one is overwhelming and confusing.
Instead, it’s more helpful to look at magnesium’s underlying physiological mechanisms. Understanding what magnesium does fundamentally will let us better comprehend how insufficient magnesium might affect our bodies and our daily lives. Magnesium’s hundreds of roles can be roughly categorized into four basic functions.
We are kept alive by trillions of chemical reactions that occur in the body. Carbohydrates are broken up and harvested for energy. New tissue is created. Cellular waste products are removed. New strands of DNA are synthesized. This collection of chemical processes is called metabolism.
The speed a reaction occurs will depend on factors like temperature, pressure, solubility and concentration of molecules. We use these factors every day. You might notice that sugar dissolves in hot water faster, or putting food in the refrigerator will slow the rate of decay. When you make a campfire, a hotter flame will burn wood faster.
Our metabolism needs to occur at a certain speed to stay alive. But we don’t have the liberty of turning the body into a raging furnace to speed up these reactions (not without damaging many things). That’s where enzymes come in.
Enzymes are bits of protein that catalyze and regulate almost all metabolic reactions. As catalysts, they reduce the energy needed to spark a chemical reaction and speed up reactions. Without enzymes, reactions that would normally take milliseconds might take hours or days.
 How long would reactions take if they proceeded spontaneously without the presence of enzymes? Dr. Richard Wolfenden, an alumni professor of biochemistry & biophysics at the University of North Carolina, posed this question. In a 1995 study, Wolfenden reported that without enzymes, the process of synthesizing DNA and RNA would take 78 million years. A subsequent study in 2008 found that producing cellular hemoglobin in the absence of enzymes would be thirty times slower, with a half-life of 2.3 billion years. That’s half the age of Earth!
How long would reactions take if they proceeded spontaneously without the presence of enzymes? Dr. Richard Wolfenden, an alumni professor of biochemistry & biophysics at the University of North Carolina, posed this question. In a 1995 study, Wolfenden reported that without enzymes, the process of synthesizing DNA and RNA would take 78 million years. A subsequent study in 2008 found that producing cellular hemoglobin in the absence of enzymes would be thirty times slower, with a half-life of 2.3 billion years. That’s half the age of Earth!
Some enzymes require an additional ions or molecules called cofactors to function. Without a cofactor bound to its structure, an enzyme may float dormant, unable to catalyze any reactions. Magnesium is a cofactor for several important enzymes in the body, like DNA/RNA polymerases, used to transcribe new DNA/RNA strands, and guanylate cyclase, used to regulate the movement of minerals across cell membranes.
A 1968 estimate suggested that magnesium was a required cofactor for 300 enzymatic reactions. This figure is found in many medical texts and quoted by many scientific papers. Since then, many more enzymes that rely on magnesium have been identified. A search of today’s enzymatic databases reveals over 600 enzymes that magnesium is a cofactor for, and another 200 enzymes that need magnesium to be activated.
In cellular metabolism, a single glucose molecule will yield 36 units of ATP.
Arguably the most important enzymes that magnesium is a cofactor for are the ones that produce cellular energy. These enzymes form a series of pathways (glycolysis, Kreb’s cycle and phosphorylation) that convert organic compounds like glucose sugars into smaller molecules called ATP, or adenosine triphosphate. ATP acts as our main unit of cellular energy.
 Every one of our hundred trillion cells manufactures ATP to store and shuttle intracellular energy. ATP stores a tremendous amount of potential energy in the bonds of the second and third phosphate groups. When the cell wants to carry out a function like cellular division or transporting molecules across the cell membrane, it breaks this bond, releasing the energy. We use a tremendous amount of ATP all the time. To get an idea of just how much we use, let’s look at some back-of-the-napkin calculations. (If you’d like to avoid the math, feel free to skip ahead!)
Every one of our hundred trillion cells manufactures ATP to store and shuttle intracellular energy. ATP stores a tremendous amount of potential energy in the bonds of the second and third phosphate groups. When the cell wants to carry out a function like cellular division or transporting molecules across the cell membrane, it breaks this bond, releasing the energy. We use a tremendous amount of ATP all the time. To get an idea of just how much we use, let’s look at some back-of-the-napkin calculations. (If you’d like to avoid the math, feel free to skip ahead!)
Assume a typical adult needs to eat approximately 2500 calories of food every day. That’s equivalent to consuming 10,460kJ of energy from our food. Let’s also assume all the metabolic pathways that convert food into ATP energy are about 50% efficient. So of the 10,460kJ of food energy we consume, 5230kJ ends up as ATP. One mole of ATP releases around 50kJ of energy in our cells, meaning the body goes through 5230/50 = 104.6 moles of ATP every day. How much is that by weight? One mole of ATP is 507 grams. 104.6 moles x 507 grams/mole = 53,032 grams or 53kg of ATP processed every day.
Our calculations estimate that 53kg of ATP is used every day. That’s a lot of ATP, about three-quarters the body weight of your average adult human! Luckily, humans are really good at recycling and recharging spent ATP (adenosine diphosphate or ADP) through those previously mentioned metabolic pathways. The typical adult only stores about 50g of ATP in the body so each ATP molecule is recycled over a thousand times daily! Since these pathways are magnesium dependent, we need quite a bit of magnesium on hand to fuel a continuous production of ATP.
 If you’re still confused about how ATP production works, think of a cell as an arcade. The different functions in your cell are like different arcade games you can play. To play any of these games, you need to insert a quarter. ATP is like that quarter. The cellular respiration pathways that magnesium assists kind of act like change machines, turning larger food compounds like glucose into ATP. A single glucose molecule after going through this change machine can yield up to 36 ATP.
If you’re still confused about how ATP production works, think of a cell as an arcade. The different functions in your cell are like different arcade games you can play. To play any of these games, you need to insert a quarter. ATP is like that quarter. The cellular respiration pathways that magnesium assists kind of act like change machines, turning larger food compounds like glucose into ATP. A single glucose molecule after going through this change machine can yield up to 36 ATP.
For those who need a brief refresher, DNA, or deoxyribonucleic acid, is a long, twisting double-stranded molecule found in the nucleus of every cell. These nucleic acids contain the sum of genetic information that makes us unique organisms. They’re also the blueprints for making all proteins in the body.
DNA strands are made of sequences of nucleotide bases: adenine, thymine, cytosine and guanine. Because of the way these nucleotides are attracted to one another, the opposite strand in a DNA molecule will have a mirror sequence of nucleotide bases. Adenine is always paired across thymine, and cytosine is always paired across guanine. These base pairs look something like this:
Magnesium is involved in the following DNA functions.
When a protein needs to be created, specific DNA nucleotide sequences are read and copied (transcribed) onto another molecule called RNA. The RNA strand is then moved out of the nucleus where enzyme-like organelles called ribosomes use it as a guide to synthesize chains of amino acids that form the desired protein.
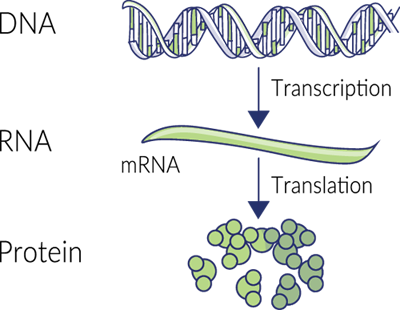
This protein synthesis relies on all sorts of enzymes to work, from helicases that open up the DNA strand to be read, to RNA polymerases that create RNA-based on the original DNA sequence, to protein kinases. Magnesium is a cofactor for most of these critical enzymes. The ribosome, while not technically an enzyme, is the most important catalyst for stitching together amino acids into proteins. Lots of magnesium is needed to keep this complex ribo protein stable.
Without enough magnesium, protein synthesis is impaired. And since protein is used for most of the structural components and nearly all metabolic functions in the body, a lack of proteins can have widespread consequences.
We mentioned earlier that magnesium is an essential cofactor for an enzyme called DNA polymerase which repairs and replicates strands of DNA using spare nucleotides floating around in the nucleus.
After other enzymes “unzip” the double-strands of DNA, DNA polymerases attach to each strand and begin to travel down the sequence of nucleotides. As it travels, the magnesium ions bound to the enzyme help open up the nucleotide site, drawing the new, free nucleotide into position.
DNA polymerase is used all the time in DNA repair and DNA copying, creating new strands at a speedy rate of 3,000 nucleotides per minute. Consider the magnitude of the role this enzyme plays. Hundreds of billions of cell divisions occur in the body daily, and each time a cell divides, it needs to replicate an identical set of DNA, or approximately 3 billion base pairs.
DNA polymerase has two binding sites for magnesium. Without magnesium, it cannot work. This is corroborated by studies that show DNA synthesis visibly slowing in the absence of enough magnesium.
DNA polymerase is a very accurate enzyme, making less than one mistake in a billion base pairs. But even if DNA is copied perfectly, mistakes in the DNA sequences do occur. Genetic damage can occur because of thermal changes, radiation, viruses or the presence of highly reactive chemicals. There’s a lot that can go wrong when you’re maintaining 3 billion base pairs. If left unchecked, these mutations will be propagated with every cell division.
There is a whole other set of processes dedicated to identifying and correcting damaged DNA. The involved enzymes cut away the damaged sections and repair the gap with fresh nucleotides. Unsurprisingly, magnesium is involved in almost every enzyme in this process.
Magnesium also has a stability effect on the structures of proteins and DNA. You might remember that for electrical charge, opposites attract and likes repel. No? Try rubbing two balloons against your hair. Now put them side by side. Because you’ve given them the same electrostatic charge, they will push apart. DNA is like that too.

Each strand in a DNA double-helix is negatively charged. Without the hydrogen bonds of their nucleotide base pairs holding them together, they will repel and break apart. In situations where DNA is exposed to higher temperatures or extreme pH, these hydrogen bonds can break. Magnesium ions have a strong positive charge.
Concentrated in the nucleus of cells, these ions can help reduce the negative charges in the DNA strands, stabilizing their structure.
This effect has been tested experimentally – a higher concentration of magnesium will measurably raise the melting temperature of DNA molecules.Many proteins and protein complexes incorporate magnesium into their structure – about 3751 human proteins with magnesium binding sites to date.
Some of those 3751 proteins dot the surface of our cell membranes, performing a variety of roles like receiving signals from hormones (signal transduction), enzymatic activity, and transporting things across the membrane. In particular, magnesium-dependent proteins are used to facilitate the transport of different minerals into and out of cells, acting as gates for sodium (Na+), potassium (K+) and calcium (Ca+).
Many of these are active transporters, for instance pumping sodium out of cells even though it’s against the concentration gradient.
Think of a flooding basement. It’s raining and water naturally flows downhill. That’s why it is leaking into the basement through cracks in the wall.
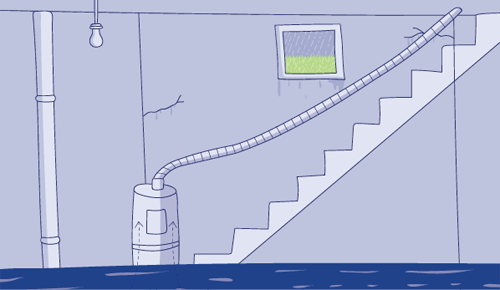
But if you have a water pump, you will be able to pump that water back out of the basement, against the gradient of gravity.
That’s why we see much greater concentrations of sodium in the extracellular environment compared to the concentrations inside cells (and vice-versa for potassium). Magnesium-powered ion pumps maintain those specific concentrations. Ionic gradients serve various purposes in the body and cells. Different cellular actions are governed through varying the concentrations of these minerals.
Sodium and potassium gradients are key to how nerve cells transmit electrical signals. When a cell receives a stimulus, the cell opens gates that allow sodium ions to rush into the cells and potassium ions to rush out. This action in one part of the cell membrane will cause nearby parts of the cell membrane to act as well, creating a travelling wave of depolarization. This wave is called the nerve impulse.
Without enough magnesium, the active transporters are unable to restore the original concentrations of sodium and potassium in the cell. This, along with a few other functions magnesium plays, can lead to an overactive nervous system which is more sensitive to random stimuli. In real life, that might translate to increased sensitivity to noise, irritability, migraines, twitching, irregular heartbeats and anxiety.
If left unchecked, a magnesium deficiency can also lead to a potassium deficiency, as potassium is released into the bloodstream and flushed out in urine.
Calcium is used in cells as a cofactor for a variety of energetic functions including nerve impulses (like sodium and potassium), cell movement and most notably muscle contractions. Because calcium is typically an excitatory cofactor, the mineral usually enters a cell only when needed for something specific, like a nervous impulse or a muscle contraction. After such an action occurs, magnesium helps active transporters pump calcium out of a cell.
Like with sodium and potassium pumps, insufficient magnesium may prevent calcium pumps from working. Unable to flush out calcium, the cell may become overstimulated, damaging the cells and even leading to cell death (apoptosis). Over excitation in nerve or muscle cells might manifest as muscle spasms or twitching, and over time can cause neurodegenerative diseases.
The next time you are doing a high-intensity workout at the gym, see if you experience any muscle cramps. Those might be an acute sign your muscle cells not being able to restore calcium balance. Take some magnesium and see what happens. Muscle contraction is the classic example of how magnesium and calcium balance each other in the body and is the most easily observable.

And it’s not just contractions in your biceps. Magnesium-regulating calcium will affect the strength of heart and arteriole contractions too. Tension headaches are caused by too much muscle tension or contraction in the head and neck.
When we pull back from the microscope and start to sketch out magnesium’s role throughout the body, we see that it is a staggering list. Here are just a few examples of conditions that magnesium is involved in.
Like other muscles, the esophageal sphincter that separates the stomach from the esophagus needs magnesium to function properly. Without enough magnesium, the sphincter may spasm, allowing the acid to travel up and irritate the sensitive esophageal lining.
Some preliminary studies link low magnesium levels with childhood ADHD. Magnesium helps reduce hyper excitability in the central nervous system by regulating concentrations of calcium, potassium and sodium via ion channels in nerve cells. It’s also a cofactor for inhibitory neurotransmitters like GABA.
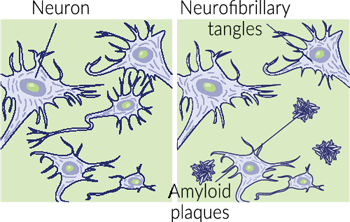
As previously mentioned, magnesium is essential for various neuronal functions. Magnesium is a cofactor for enzymes that break down and prevent the formation of amyloid-beta plaques. These plaques, commonly associated with dementia, disrupt the neuron function. A review of studies
showed that people with Alzheimer’s had lower magnesium levels in their cerebrospinal fluid than those who did not have the disease.
There are several ways magnesium helps calm the nervous system. It helps stabilize the membranes of nerve cells, regulates mineral concentrations used for nerve transmission through receptors like NMDA, is a cofactor for GABA, our body’s primary inhibitory neurotransmitter, and promotes serotonin production. It also serves several functions in our endocrine system, through the thyroid and adrenal glands. Without these inhibitory actions, our brains cannot relax, leading to anxiety, stress or panic attacks.
Inflammation is the immune system’s response to infection and injury. However, when left unchecked, systemic inflammation can lead to many different chronic conditions like cardiovascular or joint problems. While the exact mechanism of action is unknown, magnesium is thought to be an anti-inflammatory agent because levels of magnesium in the body are inversely correlated to levels of inflammatory markers like CRP and IL-6.
Clotting is a normal result of blood vessel damage. Tiny blood cells called platelets are activated when blood vessel walls are
damaged. These platelets adhere to a damaged surfaces and release sealing agents like fibrin. Magnesium regulates the activation of these platelets by controlling calcium levels and maintaining cell receptors. That’s why magnesium is sometimes called an anticoagulant.
Magnesium deficiencies increase the risk of unnecessary platelet activation, forming more clots in blood vessels. These clots may block blood flow to the brain or heart, increasing the risk of heart attack and stroke.
 Besides its role in preventing blood clots, magnesium also acts as a natural vasodilator. Magnesium, as a calcium antagonist, allows the heart muscles and the smooth muscles of the arteries to rest and relax, reducing blood pressure. If there is insufficient magnesium, these blood vessels constrict, raising blood pressure.
Besides its role in preventing blood clots, magnesium also acts as a natural vasodilator. Magnesium, as a calcium antagonist, allows the heart muscles and the smooth muscles of the arteries to rest and relax, reducing blood pressure. If there is insufficient magnesium, these blood vessels constrict, raising blood pressure.
 Like elsewhere in the body, magnesium regulates concentrations of potassium and calcium in the heart. Those concentrations control and coordinate the rhythm of electrical signal and muscle contractions. Following recommendations by the Canadian Cardiovascular Society, hospitals administer magnesium intravenously to reduce the risks of atrial fibrillation and other irregular heartbeats.
Like elsewhere in the body, magnesium regulates concentrations of potassium and calcium in the heart. Those concentrations control and coordinate the rhythm of electrical signal and muscle contractions. Following recommendations by the Canadian Cardiovascular Society, hospitals administer magnesium intravenously to reduce the risks of atrial fibrillation and other irregular heartbeats.
Magnesium improves insulin sensitivity, allowing the body to use and store the glucose it ingests and to excrete excess glucose. A magnesium deficiency can cause a sluggish insulin response to blood sugar, leading to insulin resistance and type 2 diabetes.
 Magnesium doesn’t only regulate calcium in cells. It stimulates the production of calcitonin, the hormone responsible for inhibiting cells (osteoclasts) that breaks bone down and activates alkaline phosphatase, an enzyme that forms new calcium crystals in bones.
Magnesium doesn’t only regulate calcium in cells. It stimulates the production of calcitonin, the hormone responsible for inhibiting cells (osteoclasts) that breaks bone down and activates alkaline phosphatase, an enzyme that forms new calcium crystals in bones.
If your vitamin D levels are low, you might be lacking in magnesium, too. Magnesium regulates key enzymes that convert the vitamin D into the active form used by the body (1,25OHD). No matter how much vitamin D you get from the sun or from your diet, insufficient magnesium will mean insufficient vitamin D.
By extension, vitamin D promotes calcium absorption in the small intestine. Low vitamin D status can prevent enough calcium from being absorbed.
Magnesium is vital for the proper function of sodium-potassium pumps in the cell membranes, keeping potassium inside cells at high concentrations and potassium in the blood at low concentrations.
Without enough magnesium working these ionic pumps, too much potassium is released into the bloodstream where it is flushed out in urine.
Magnesium is important for bowel peristalsis – the contraction and relaxation of intestinal muscles that move food down the intestinal tract.
In addition to regular neural activity, magnesium helps to convert tryptophan into serotonin. Serotonin is a neurotransmitter thought to maintain mood balance. Low levels of serotonin may lead to depression.

Seizures are caused by abnormal electrical activity in the brain. Trauma, severe stress or conditions like epilepsy can cause neurons to fire excessively. Magnesium helps to stabilize cell membranes in nerve cells, preventing them from misfiring.
Insufficient magnesium to regulate calcium may cause the stapedius muscle in the ears to contract and remain tense. This tension combined with overexcited nerve firings may cause sensitivities to loud noises.
Magnesium is linked to headaches and migraines in a few ways. Magnesium allows muscles to relax properly. Without it, muscles are prone to tense up or cramp. Muscle tension in the neck and head may cause headaches. Insufficient serotonin, which magnesium helps produce, can result in migraines, as can small blood clots reducing blood flow to the brain.
Considering magnesium has a calming effect on the mind, it’s a little counterintuitive that magnesium is also involved in countering fatigue. Magnesium works on multiple levels when combating low energy fatigue. Magnesium is involved in metabolizing glucose into available energy, restoring ATP to depleted muscles, and regulating stress hormones.
Insufficient magnesium causes calcium to stay in muscle and nerve cells longer, causing over excitation of the muscles and nerves. This can lead to cramps, spasms, and other muscle related issues.
Magnesium is involved in the production of melatonin, a hormone that helps control sleep and wake cycles. Muscle tension due to lack of magnesium will also prevent good sleep.
Magnesium aids in the manufacture of hormone proteins progesterone and estrogen. During times of elevated progesterone and estrogen, more magnesium is used, leaving a potential shortage. Insufficient magnesium may cause typical PMS symptoms like insomnia, irritability, cramps, bloating and tenderness.
Preeclampsia typically manifests as a sharp rise in blood pressure during the late stages of pregnancy. If seizures occur, then it is known as eclampsia. As with other cardiovascular conditions, magnesium is used to treat this (usually through IV) and is thought to relax the blood vessels, thus decreasing the blood pressure.
Magnesium plays a bronchodilating effect, increasing airflow to the lungs. Part of this is due to magnesium’s anti-inflammatory properties, as inflamed bronchioles can prevent the exchange of oxygen to the blood. Magnesium also inhibits the release of histamines, which are known to constrict the bronchi and bronchioles.
Magnesium deficiencies can hinder endurance in sports or fitness. Muscles have limited quantities of ATP that need to be replenished during activity. Magnesium helps to recycle that ATP. Magnesium also helps with proper muscle relaxation and contraction.
Learn about how magnesium works, why it’s important, and how it can help you.

Necessary cookies are absolutely essential for the website to function properly. This category only includes cookies that ensures basic functionalities and security features of the website. These cookies do not store any personal information.
Any cookies that may not be particularly necessary for the website to function and is used specifically to collect user personal data via analytics, ads, other embedded contents are termed as non-necessary cookies. It is mandatory to procure user consent prior to running these cookies on your website.
These cookies collect information about how visitors use a website, for instance which pages visitors go to most often, and if they get error messages from web pages. These cookies don’t collect information that identifies a visitor.
The “Other” category cookies help us provide our visitors proper functionality of the website such as online quizzes or embedded videos.
Analytics cookies help us understand how our visitors interact with the website. It helps us understand the number of visitors, where the visitors are coming from, and the pages they navigate. The cookies collect this data and are reported anonymously.
Advertisement cookies help us provide our visitors with relevant ads and marketing campaigns.