LOWELL, Mass., June 29, 2020 -- Versatope Therapeutics, Inc, a biotechnology company developing vaccines and therapeutics, announced it is expanding its platform to develop a coronavirus vaccine for multiple strains. In addition, the company has progressed on an expanded vaccine pipeline to be announced in 2020.
The current SARS-CoV-2 pandemic has brought attention to the urgent need for a rapid vaccine response against pandemic infectious diseases.
For years, scientific researchers from universities, small companies, and even global pharmaceutical organizations and business leaders have been screaming this into a vacuum. See Bill Gates’ TED Talk in 2015 warning on the next global pandemic. Today, biopharma investors are hearing the call and recognizing the urgency to develop vaccines for pandemic infectious diseases.
Companies with legitimate vaccine development platforms are squarely in focus for biopharma investment. Currently, most investors are keenly focused on the development of a vaccine for COVID-19. Moderna, Inc. (MRNA) has been the first to clinic, subsequently seeing its stock soar more than 150% YTD on the recent progress of the company’s mRNA vaccine candidate, mRNA-1273, against SARS-CoV-2.
In April 2020, Moderna was awarded $483 million from the Biomedical Advanced Research and Development Authority (BARDA) to accelerate the development of mRNA-1273; and, Moderna raised another $1.34 billion from investors. BARDA has been aggressively funding vaccine development for COVID-19. In March, BARDA awarded over $1 billion to Johnson & Johnson (JNJ) to help the company scale up manufacturing capacity for its COVID-19 vaccine candidate. The collaboration of Sanofi-Glaxo received BARDA funding in April 2020, and AstraZeneca (AZN) and Oxford University announced BARDA has committed over $1 billion to help fund the development of its COVID-19 vaccine candidate, AZD1222.
Companies with more traditional vaccine development platforms for coronavirus, as well as those with novel approaches, are doing well in anticipation of increased valuation. Investors should seek out other vaccine companies with validated plug-and-play platforms capable of rapid vaccine development.
The Power of Versatope’s Vesicle Platform
One such potential company is privately-held Versatope Therapeutics. Versatope’s synthetic biology platform uses exosome-like vesicles, a commercially proven technology with significant product development potential. These vesicles are nature’s liposomes, tiny outer membrane-bound bubbles that are highly immunogenic when used to deliver vaccines and can also be genetically engineered for the targeted delivery of large molecules used in therapeutics and other modalities. They are highly customizable and can be delivered by injectable, oral, and mucosal routes of administration.
Versatope’s exosome-like vesicles – also called recombinant outer membrane vesicles (rOMVs) - can be genetically engineered to express surface antibodies, receptors, and antigens to target specific diseases. The company’s initial vaccine asset is a universal flu vaccine that expresses genetically-conserved proteins found on the influenza virus. This is in stark contrast to the “pick-a-strain” strategy for all of the commercially-available flu vaccines we have today - a strategy that the U.S. CDC says resulted in only 10-60% effectiveness over the past 15 years.
Versatope’s concept is simple: train the body to recognize and attack part of the virus critical for its survival - the Matrix-2 (M2) ion channel, and one of the regions that remains constant from year to year, thereby hitting it with a robust immune response. The technology is based on research from Cornell University with an exclusive worldwide license to Versatope. Cornell’s preclinical data shows survival against lethal doses of the influenza strains of avian H1N1, swine H3N2 and pandemic H5N1.
This potent protection is driven largely via an antibody-mediated response that targets virus infected cells with active influenza infection, rather than blocking viral attachment to host cells. The goal is to take the guesswork out of the flu vaccine development and create a “one-and-done” product that vastly reduces the infection rate and prevents millions of hospitalizations and the over 650,000 deaths from the flu each year, as well as an estimated $16B in lost earnings in the U.S. It’s a concept that the NIH and NIAID liked enough to award Versatope with $17.9 million in initial funding in September 2019.
The worldwide flu vaccine market hit $5.2 billion in 2018 and is expected to grow to $7.5 billion by 2024. It is dominated by global pharmaceutical behemoths like Sanofi, Glaxo, AstraZeneca, and CSL. Israel-based BiondVax Pharmaceuticals (BVXV) is currently conducting a large Phase 3 clinical development with its universal flu vaccine candidate, M-001. BiondVax shares have quadrupled in the past two months and the company currently sports a market value of $240 million.
UPDATE: Intravacc and Versatope Sign Research Service Agreement to Develop Universal Influenza Vaccine Based on OMV technology.
Versatope’s Competitive Advantages: Developing a Pan-Coronavirus Vaccine
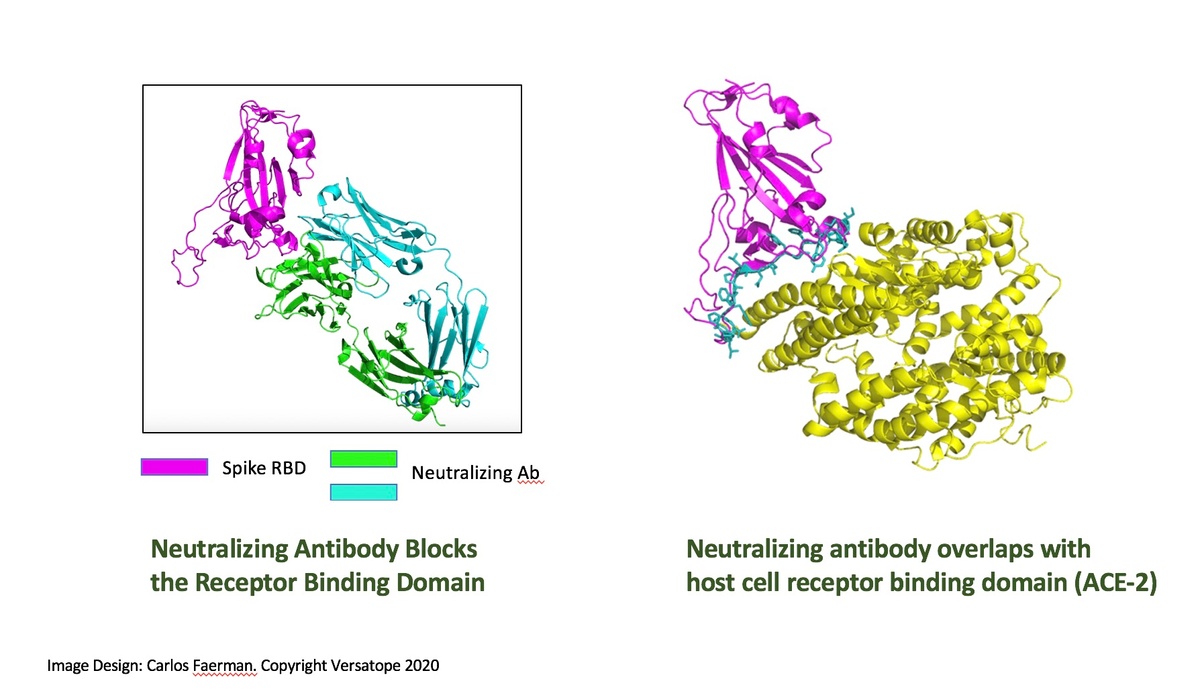
Investors should particularly take note of the Versatope’s potential competitive advantage in their ability to design a pan-coronavirus vaccine (including COVID-19) based on key findings from their influenza studies. The company’s products are derived from a probiotic strain of E. coli that are safe and amenable to rapid modification through genetic engineering. This allows for the preparation of highly customizable rOMV candidates, not only in the influenza vaccine market but in other areas of infectious disease as well, such as COVID-19. Antigens can be expressed not only to the spike protein that is the target of vaccines such as mRNA-1273 and AZD1222, but also to other important regions of the SARS-CoV-2 virus. The company is currently seeking funding for the advancement of its pan-coronavirus/COVID-19 vaccine development.
Commercial Validation of Versatope’s rOMV Platform
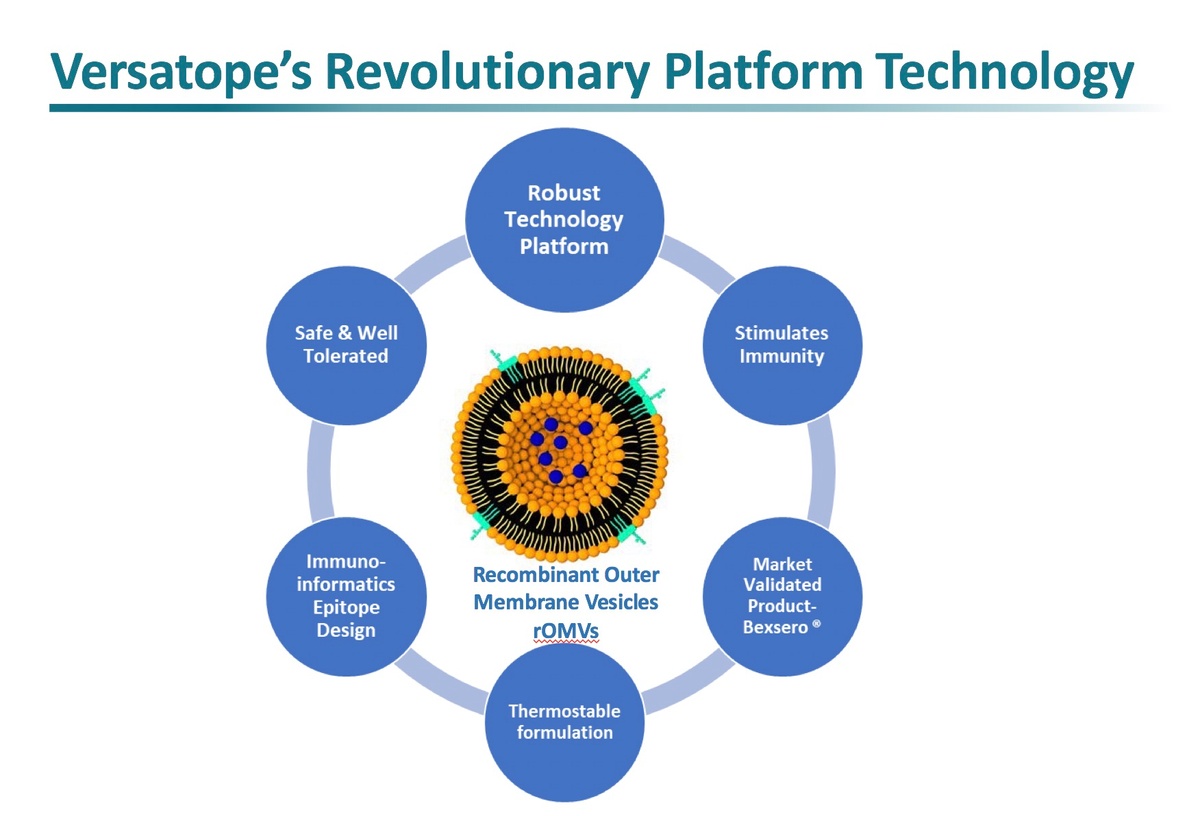
Commercial manufacturing for rOMV products has been well-established, as validated by Glaxo’s meningococcal group B vaccine, Bexsero®, launched in 2013 and is projected by EvalutePharma to have over $1 billion in worldwide sales in 2020. However, Versatope’s rOMV has advantages over products like Bexsero® because it is engineered from a detoxified BSL-1 probiotic strain of E. coli that has a crippled form of endotoxin to minimize reactogenicity. This not only eliminates the extra step of chemical lipopolysaccharide (LPS) detoxification, but also reduces costs.
Large scale and favorable cost manufacturing are additional potential advantages of Versatope’s platform. Importantly, these products are stable and have the potential for continuous manufacturing, lyophilization and spray-drying to avoid a refrigerated supply chain and reduce the cost of goods.
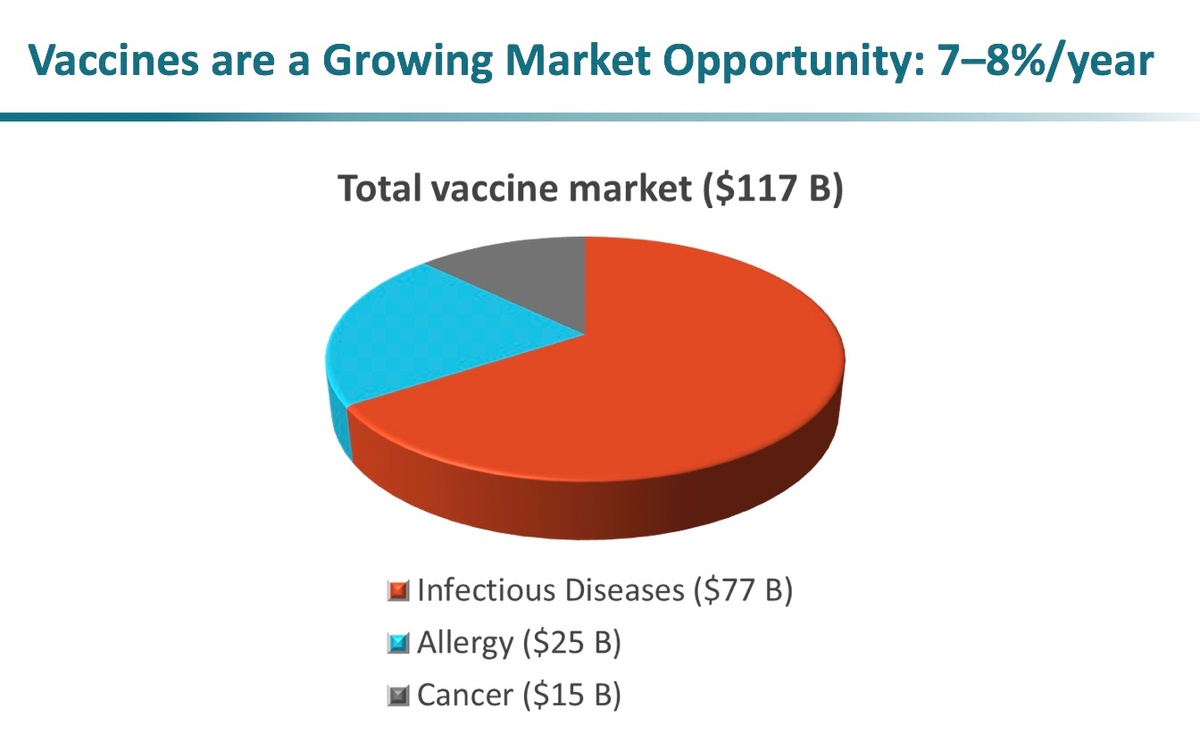
Potential for Targeted Immunity for Oncology and Allergy
Multiple targets can be expressed using positional assembly that can have a broad impact for protection in infectious disease, and can also be broadly applicable in immuno-oncology and allergy. In the area of immuno-oncology, multiple tumor-associated antigens can be expressed that potentially overcome the antigen-escape issue that has plagued single-antigen cancer vaccines in the past. In allergy, multiple allergens can be expressed on the vesicles to provide wide protection from a combination of a variety of allergies. Furthermore, the applicability of Versatope’s platform goes beyond even these areas and expands into cell targeting, gene/drug delivery, biosensing, and bioremediation.
Conclusion
Versatope’s transformational technology using synthetic biology offers the potential for strong competitive advantages for vaccine development, including capturing genetic diversity, rapid development and broad-scale immunologic flexibility. Safety, thermostability and manufacturing efficiency are additional benefits of the technology that make it unique and ideal for a pan-coronavirus vaccine and emerging pandemics.
Versatope is already advancing on the potential best-in-class universal flu vaccine and with additional funding, the company will expand into new areas beyond pandemic infectious diseases into oncology and allergy.
To learn more about how Versatope is developing a pan-coronavirus vaccine with their transformational synthetic biology technology: Get in Touch













