Medicines for Addiction
Adial is a clinical-stage biopharmaceutical company focused on the treatment and prevention of addictions and other unmet medical needs.
RECENT NEWS
Adial Pharmaceuticals Reports 2024 Fiscal Year Financial Results and Provides Business Update
March 4, 2025
GLEN ALLEN, Va., March 04, 2025 — Adial Pharmaceuticals, Inc. (NASDAQ: ADIL) (“Adial” or the “Company”), a clinical-stage biopharmaceutical company focused on developing therapies for the treatment and prevention of addiction and related disorders, today provided a business update and reported its financial results for the 2024 fiscal year ended December 31, 2024. Key Highlights […]
HEAR FROM OUR EXPERTS
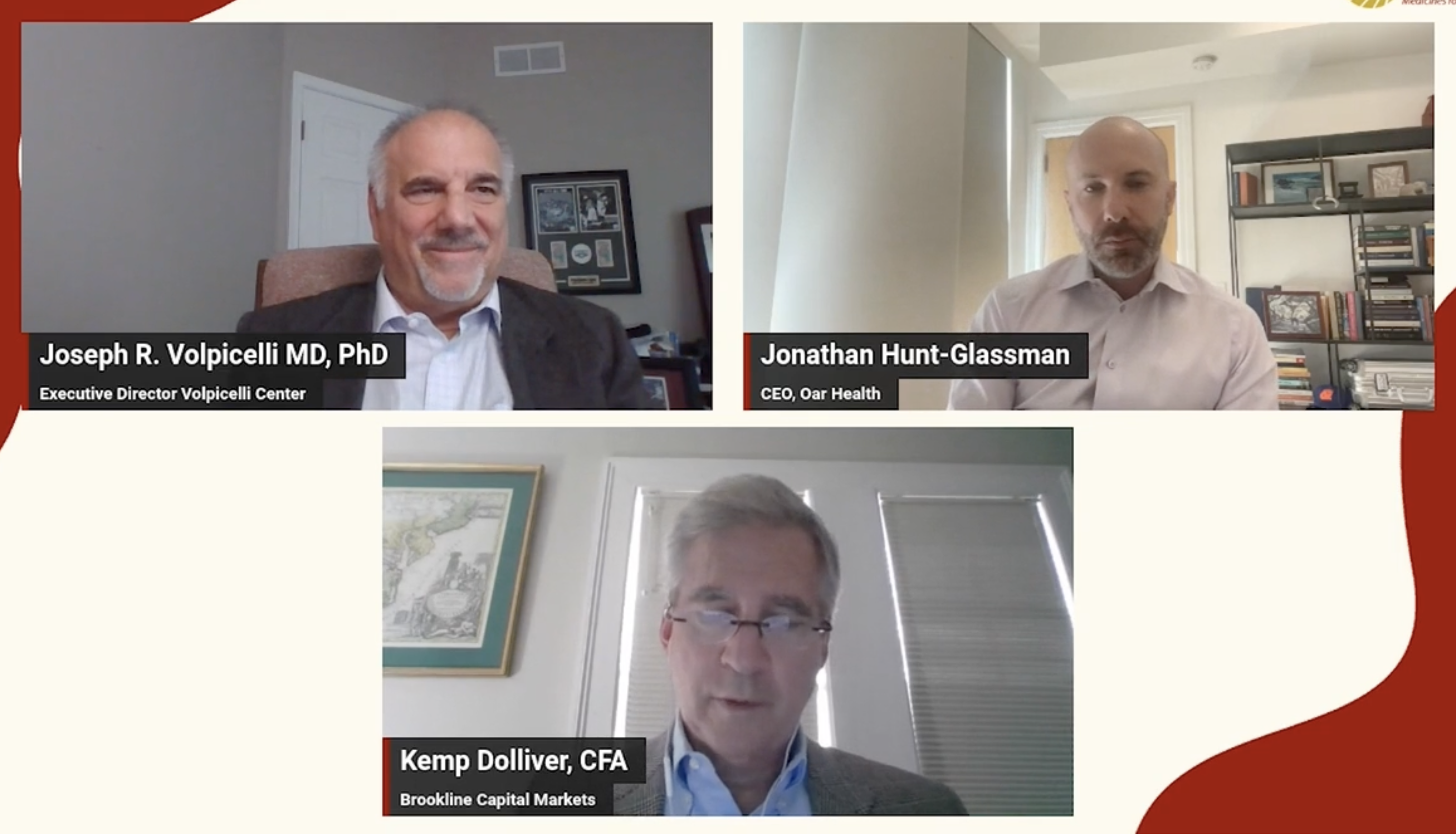
Addiction Experts Highlight the Need for Non-Abstinence-Based Therapies to Treat Alcohol Use Disorder in Adial Pharmaceuticals Key Opinion Leader Webinar.
Key Opinion Leader WebinarManaging Through Mixed Trial Results And An Economic Downturn
Ben Comer
Chief Editor, Life Science Leader
November 1, 2022
What would Jack Welch do? Cary Claiborne knows the answer from nearly 15 years working at GE and GE Capital, during a time that overlapped closely with Welch’s tenure as CEO. In August, Claiborne himself became CEO of Adial Pharma, a Charlottesville, VA-based biopharmaceutical company with a novel approach to treating alcohol use disorder (AUD). Claiborne faces several distinct challenges: mixed topline results from a Phase 3 trial of lead candidate AD04, the potential need to fund and conduct another trial prior to seeking regulatory approval, and managing the company through a difficult economic downturn. Lessons learned during formative work experiences at GE, the first place Claiborne worked after graduating from Rutgers University in 1982, have followed him through executive-level financial roles in industries including telecoms, retail, energy, renewable fuels, and biopharmaceuticals.

Spotlight Series
Stay updated with insights from Adial’s executives on the latest company news and key developments in addiction research.
View All Spotlight SeriesFrom Data to Recovery: Transforming AUD Treatment with Precision Medicine
01/29/2025
Adial Pharmaceuticals is leveraging genetic insights to redefine AUD care with targeted, patient-specific solutions Since its inception, Adial Pharmaceuticals has recognized the potential of precision medicine in treating Alcohol Use Disorder (AUD). Built on a commitment to scientific innovation, Adial has dedicated itself to developing therapeutic interventions that take into…
read more →AD04
Our lead investigational drug candidate, AD04, is a genetically targeted, serotonin-3 receptor antagonist, therapeutic agent for the treatment of Alcohol Use Disorder (AUD) and is currently being investigated for the potential treatment of AUD in patients with certain target genotypes, which are to be identified using the Company’s proprietary companion diagnostic genetic test.
Learn MoreInvestors
Learn MoreNasdaq: ADIL
Price
Volume