Manufacturing more tomorrowsTM
Innovative CDMO solutions
From development to commercial manufacturing and beyond, amplify your product’s impact with our exceptional-by-design CDMO solutions.

Connect with us
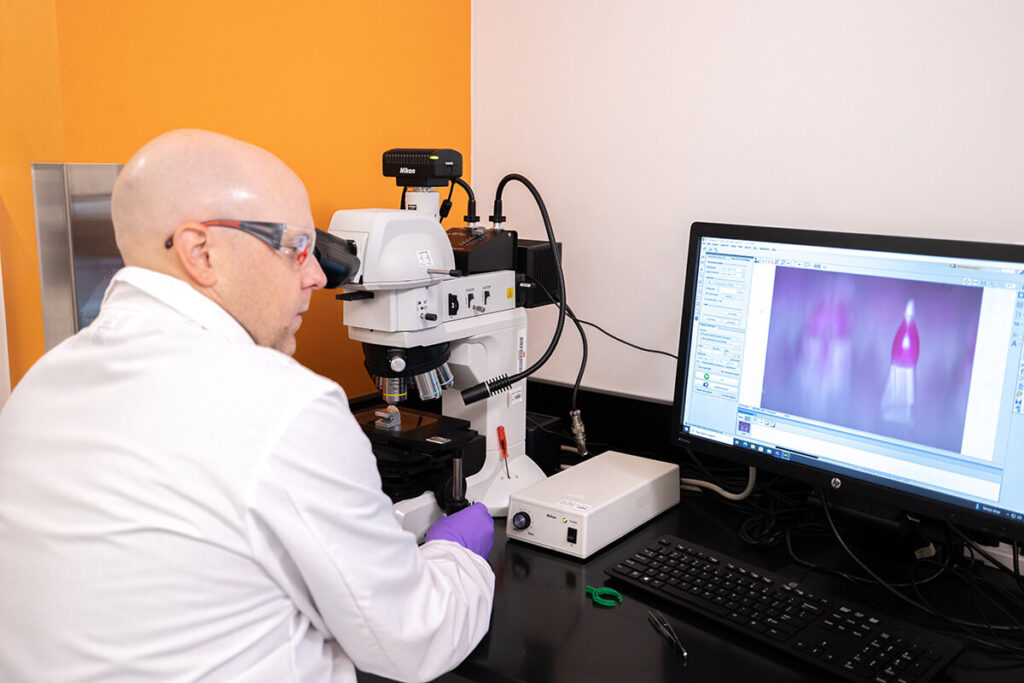
Our Bridgeton facility is a state-of-the-art center for aseptic operations, featuring advanced isolator and automation technology to ensure the highest levels of quality and patient safety.
We’re hiring
Join us and become part of a CDMO dedicated to advancing our clients’ projects, ambitions and our industry.
Our legacy of innovation
History of Kindeva
Since 1956, we have been developing technologies that meet the demands of today and deliver the possibilities of tomorrow.

1956
Invented the pressurized metered‑dose inhaler (pMDI)
Invented the pressurized metered‑dose inhaler (pMDI) and kick-started the evolution of inhaled therapies by developing an easy-to-use device for patients around the globe.

1959
Created the emergency use autoinjector
Created the emergency use autoinjector, laying the groundwork for the ongoing refinement of self-administered injectables currently used by millions of people worldwide.

1970
Launched the drug-in-adhesive patch
Launched the drug-in-adhesive patch, providing a noninvasive method for delivering a drug over an extended timeframe.

1970
Invented the ComboPen® platform
Invented the ComboPen® platform to deliver medical countermeasures, which fueled autoinjector innovations for anaphylaxis.

1989
Developed the breath-actuated inhaler
Developed the breath-actuated inhaler, a lifesaving option for individuals with hand-breath coordination problems that make traditional inhalers difficult to use.

1995
Launched the CFC-free MDI
Launched the CFC-free MDI and set the standard for future care options developed to help safeguard the environment.
2002
Invented the BinaJect® platform
Invented the BinaJect® platform, bringing an industry-leading dual-chamber autoinjector option to market with enhanced stability and bioavailability.

2005
Solid microneedle array patch development
Solid microneedle array patch development, creating accurate, reliable intradermal delivery with the potential to eliminate cold-chain storage and enhance immunogenicity and efficacy.

2009
Created the TruJect™ platform
Created the TruJect™ platform and introduced the next generation of easy and reliable single-chamber autoinjectors, widely used to deliver a variety of medications.

2010
Developed the first commercially available dose counter
Developed the first commercially available dose counter, ensuring patients knew the number of inhaler actuations remaining so they could always be prepared.

2012
Invented the CFC-free nasal MDI
Invented the CFC-free nasal MDI, a more environmentally sound device for drug delivery capable of bypassing the blood-brain barrier.

2024
Developing low-GWP inhalers
Developing low-GWP inhalers and planning the opening of one of the first commercial-scale green propellant lines for filling inhalers using propellants with up to 99.9% lower GWP than current options.
Let’s transform tomorrow together
Every patient deserves a brighter tomorrow. As your strategic partner, we are dedicated to building your lasting legacy and helping you fast-track healthier tomorrows. You dream it, we deliver it.
Latest at Kindeva
Tomorrow’s in the making at Kindeva. Explore our latest insights and expert resources to advance your drug delivery development and manufacturing.
The three key trends that will most impact drug development in 2025
The speed of change in the drug development and manufacturing sector requires its stakeholders to be proactive in understanding and addressing key trends and directions the industry is taking. As a global market of considerable size and significance, the value of which is anticipated to grow from $589.06 billion in 2024 to $632.71 billion in […]
Learn MoreMicroneedle array patch characterization recording
Microneedle array patches (MAPs) have the potential to transform delivery of medications and vaccines. While this innovative drug delivery format could result in improved efficiency across a wide range of therapeutics, it also brings critical challenges. To successfully move from concept to commercialization, MAP manufacturers must consider scalability, regulatory compliance, and other complexities. In this […]
Learn MoreMicroneedle array patch characterization presentation
Microneedle array patches (MAPs) are a transformative innovation in drug delivery, with the potential to redefine how medications and vaccines reach patients. MAPs promise a simpler, more effective way to deliver a wide range of therapeutics, but their journey from concept to commercialization is filled with critical challenges. From scaling manufacturing to ensuring regulatory compliance, […]
Learn MoreAddressing top tech transfer challenges
Technology transfer represents a critical bridge between development and commercial manufacturing. This complex process — moving product and process knowledge between development and manufacturing teams or between different manufacturing sites — demands precision at every stage. The implications of technology transfer extend beyond knowledge sharing. Each decision during this process directly impacts manufacturing efficiency, regulatory […]
Learn MoreTech transfer commercial manufacturing mindset
Technology transfer (tech transfer) moves product and process knowledge between teams or partners and acts as a crucial bridge between ideation and commercialization. Especially for complex products like sterile injectables, a smooth tech transfer process is essential. In this white paper, discover valuable strategies to embrace a commercial manufacturing mindset from day one. Download your […]
Learn MoreHFA152a green propellant capabilities
With evolving regulations and a growing push for sustainability, switching pressurized metered-dose inhalers (pMDIs) to green propellants is becoming increasingly essential. One of the most promising low global warming potential (GWP) options is HFA152a. In this white paper, we share insights on transitioning to HFA152a. Download your copy to discover: An overview of sustainability regulations […]
Learn MoreSterile product introduction and tech transfers in a crowded market
The tech transfer landscape for sterile products is increasingly complex, with pressure to reduce time to market. Find out how to navigate common challenges and avoid costly delays or oversights in this presentation from our Director, Client Portfolio & Relationship Management, Kim Brown. Download your copy to explore: Benefits of a commercial manufacturing mindset How […]
Learn MoreEliminating the syringe with inhaled and microneedle vaccine delivery systems
Interest in inhaled and intradermal vaccine delivery systems isn’t just a modern innovation — one of history’s most effective vaccination campaigns also used mucosal or dermal delivery. In 1777, George Washington mandated smallpox inoculation for his troops using either arm scratches (intradermal) or nasal inhalation (mucosal), a strategy that proved crucial in the American victory.1 […]
Learn MoreLeading the way in low-GWP propellants
As regulations surrounding inhalation device sustainability evolve, we help you streamline the process of transitioning to low global warming potential (GWP) propellants. Clinical supply for green propellants HFA152a and HFO1234ze is already available, with commercial scale available this year and plans for further expansion. Download this information sheet to dive deeper into our low-GWP propellant […]
Learn More