Oral Mucositis Market
- The Oral mucositis market size is anticipated to grow with a significant CAGR duing the study period (2020-2034).
- Among the 7MM, the United States accounts for the highest Oral mucositis market size in 2023.
- Treatment interventions include general oral care protocols; interventions to reduce the mucosal toxicity of chemotherapy drugs; mouthwashes with mixed action; immunomodulatory agents; topical anesthetics; antiseptics; mucosal barriers and coating agents; cytoprotectants; mucosal cell stimulants; psychotherapy; and analgesics.
- The treatment options currently in the market include Wecare’s Oralcare rinse and gel, palifermin, episil, MuGard, acyclonine MUM among others.
- Oral mucositis companies such as - Amgen, Swedish Orphan Biovitrum, Access Pharmaceuticals, Chemo Mouthpiece/ Aurora BioScience, Camurus, Innovation Pharmaceuticals, Enzychem Lifesciences Corporation, BrainCool, NeoMedLight, Monopar Therapeutics, and others are focusing on developing innovative therapies to manage and treat painful inflammation caused by cancer treatments.
- Oral mucositis MOA involves chemotherapy- or radiotherapy-induced damage to the oral mucosa, leading to inflammation, ulceration, and pain due to activation of inflammatory pathways and epithelial cell apoptosis.
- In August 2023, the US FDA issued a Complete Response Letter (CRL) for avasopasem manganese for radiotherapy-induced severe oral mucositis stating that the results from the Phase III ROMAN trial are not sufficiently persuasive to establish substantial evidence of avasopasem’s effectiveness and safety. The Company intends to request a Type A meeting with the FDA to understand the FDA’s rationale for its decision and discuss the next steps to support an NDA resubmission seeking approval of avasopasem.
- In March 2023, the FDA granted a fast-track designation to RRx-001 for the prevention and attenuation of severe oral mucositis associated with chemotherapy and radiation in patients with head and neck cancer.
DelveInsight's “Oral mucositis Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of Oral mucositis epidemiology, market, and clinical development in Oral mucositis. In addition to this, the report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the Oral mucositis therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
The Oral mucositis market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted Oral mucositis market size from 2020 to 2034 in 7MM. The report also covers current Oral mucositis treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
| Study Period | 2020 to 2034 |
| Forecast Period | 2024-2034 |
| Geographies Covered |
|
| Oral Mucositis Market |
|
| Oral Mucositiss Market Size | |
| Oral Mucositis Companies | Amgen, Swedish Orphan Biovitrum, Access Pharmaceuticals, Chemo Mouthpiece/ Aurora BioScience, Camurus, Innovation Pharmaceuticals, Enzychem Lifesciences Corporation, BrainCool, NeoMedLight, Monopar Therapeutics, MuReva (Lumitex), EpicentRx/Prothex Pharma, Soligenix, and others. |
| Oral Mucositis Epidemiology Segmentation |
|
Oral mucositis Treatment Market
Oral mucositis Overview and Diagnosis
Oral mucositis (OM) is a severely debilitating condition characterized by erythema, edema, and ulcerations of the oral mucosa. Oral mucositis occurs when cancer treatments break down the rapidly divided epithelial cells lining the gastrointestinal tract, leaving the mucosal tissue open to ulceration and infection. Commonly observed signs and symptoms are red, shiny, or swollen mouth and gums; blood in the mouth; sores in the mouth, gums, or tongue; soreness or pain in the mouth or throat; difficulty swallowing or talking; feeling of dryness, mild burning, or pain when eating food; soft, whitish patches or pus in the mouth or on the tongue and increased mucus or thicker saliva in the mouth.
The Oral mucositis diagnosis is typically based on the appearance, location, and timing of oral lesions, as well as the medical history, which may show a medication or treatment form that is highly linked with OM. Useful laboratory tests for confirming diagnosis and etiology, especially if local fungal, bacterial, or viral infections infection is suspected, are complete blood count, viral culture, biopsy, and fungal testing.
Further details related to country-based variations in diagnosis are provided in the report...
Oral mucositis Treatment
Management of oral mucositis is divided into the following sections: nutritional support, pain control, oral decontamination, palliation of dry mouth, and therapeutic interventions for oral mucositis. Topical agents, analgesics, mucosal protectants, etc. are used. The oral mucositis clinical trials market is expanding rapidly due to increasing cancer therapies requiring supportive care interventions. The Oral Mucositis Drugs Market is expanding due to rising cancer therapies, increasing demand for effective supportive care treatments.
The other treatment options may include interventions to reduce the mucosal toxicity of chemotherapy drugs, mouthwashes, immunomodulatory agents, topical anesthetics, mucosal barriers and coating agents, and cytoprotectants. Rinsing the oral cavity with non-medicated oral rinses - saline water rinse, sodium bicarbonate rinse, or a combination of sodium bicarbonate and saline water- is recommended every four hours. The oral mucositis drugs for cancer therapy market is expanding rapidly, driven by increasing chemotherapy-related side effects and innovation.
Oral mucositis Epidemiology
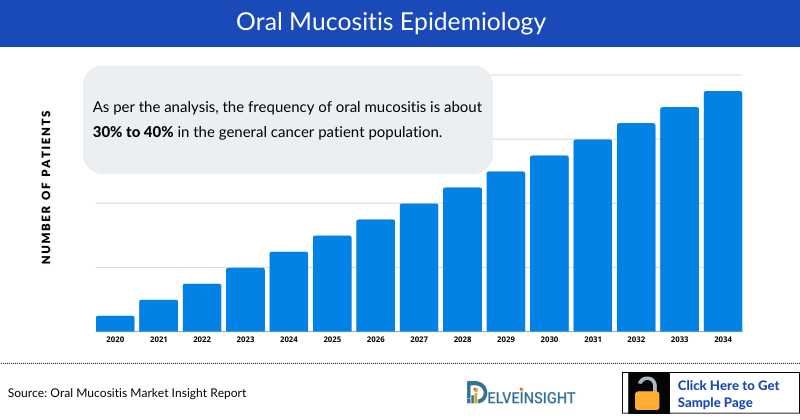
The Oral mucositis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total Incident Cases of Oral Mucositis, Grade-specific Cases of Oral Mucositis, and Total Treated cases of Oral Mucositis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- According to the Oral Cancer Foundation (2022), OM is probably the most common, debilitating complication of cancer treatments, particularly chemotherapy and radiation.
- As per the analysis, the frequency of oral mucositis is about 30% to 40% in the general cancer patient population.
- A significantly greater risk of oral mucositis has been found in women than in men.
Stay ahead with insights on Oral Mucositis prevalence and patient population projections.
Oral mucositis Drug Chapters
The drug chapter segment of the Oral mucositis market report encloses a detailed analysis of Oral mucositis marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Oral mucositis pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations. Advancements in oncology treatments are fueling the oral mucositis clinical trials market, driving innovation in pain relief and healing therapies. Oral mucositis MOA involves inflammation and ulceration caused by damage to mucosal cells during chemotherapy or radiation therapy.
Oral mucositis Marketed Drugs
KEPIVANCE (palifermin): Swedish Orphan Biovitrum
KEPIVANCE is a recombinant human keratinocyte growth factor that works at the cellular level to help protect patients with hematologic malignancies undergoing high-dose chemotherapy and/or radiation followed by bone marrow transplant from severe oral mucositis. The drug received FDA approval in December 2004 to reduce the incidence and duration of severe oral mucositis in these patients by protecting the epithelial cells that line the mouth and throat from the damage caused by chemotherapy and radiation and by stimulating the growth and development of new epithelial cells to build up the mucosal barrier.
Stay ahead with key updates on Oral Mucositis treatments. Access the 2025 pipeline report for exclusive insights!
| Table 1: Comparison of Key Marketed drugs | ||||
| Drug name | Company | RoA | MoA | FDA Approval |
| KEPIVANCE | Swedish Orphan Biovitrum | Intravenous | Programmed cell death protein (PD-1) inhibitor | 2004 |
Emerging Oral mucositis Drugs
Avasopasem manganese (GC4419): Galera Therapeutics
Avasopasem manganese is an investigational, highly selective small molecule superoxide dismutase (SOD) mimetic that is being developed for the reduction of radiation-induced severe oral mucositis. It is designed to rapidly and selectively convert superoxide to hydrogen peroxide and oxygen, protecting normal tissue from damage associated with radiation therapy. Avasopasem received Breakthrough Therapy and Fast Track Designations from the US FDA for the reduction of the incidence and severity of radiation-induced oral mucositis in head and neck cancer. Currently, the drug has completed a Phase III (NCT03689712) trial (ROMAN) to evaluate the reduction in oral mucositis with GC4419 (avasopasem manganese) if administered prior to intensity-modulated radiation therapy (IMRT) in patients receiving chemoradiotherapy for locally-advanced, non-metastatic head and neck cancer.
RRx-001: EpicentRx
RRx-00, is a direct NLRP3 inhibitor and Nrf2 up regulator that has anti-inflammatory and antioxidant properties. It inhibits glucose 6-phosphate dehydrogenase (G6PD) in human tumor cells, binds hemoglobin, and drives RBC-mediated redox reactions. It triggers apoptosis and exhibits anticancer activity. The company has completed a Phase II (NCT03515538) trial (PREVLAR) to evaluate the safety and efficacy of RRx-001 in the attenuation of oral mucositis in patients receiving chemoradiation for the treatment of oral cancers and is currently evaluating the drug in another ongoing Phase II (KEVLARx) trial for reducing oral mucositis in patients receiving chemotherapy and radiation for head and neck cancer.
Note: Detailed emerging therapies assessment will be provided in the final report...
| Table 2: Comparison of key emerging drugs | |||||
| Drug name | Company | RoA | MoA | Phase | Special Status |
| Avasopasem manganese (GC4419) | Galera Therapeutics | IV infusion | Superoxide dismutase mimetic | III | FTD, BTD |
| RRx-001 | EpicentRx | Intravenous | NLRP3 inhibitor | II | FTD |
Drug Class Insights
The drug classes commonly used to address oral mucositis include Topical Anesthetics such as lidocaine and benzocaine. Mucosal Coating Agents that form a protective barrier over the oral mucosa, reducing irritation and promoting healing. Sucralfate and coating mouthwashes fall into this category. Oral Rinses such as Wecare’s Oralcare Rinse or MuGard can help reduce the risk of infection and promote healing. Other anti-inflammatory Agents like corticosteroids are used as well. Certain growth factors, such as recombinant human keratinocyte growth factor (Palifermin), may stimulate the growth and repair of the oral mucosa. Other than these, several pain medications, depending on the severity of the pain, analgesics such as acetaminophen or opioids may be also prescribed.
Oral mucositis Market Outlook
Oral mucositis is a common and feared adverse effect in patients with cancer who undergo anticancer treatment. The management of mucositis can be quite vexing for both the patient and the oncologist. Uncomplicated mucositis is generally self-limiting, and symptom management and supportive care may be all that is needed. Normal saline or sodium bicarbonate solutions can provide relief of mild to moderate mucositis pain.10 Such salt-and-soda mouthwashes are also safe, inexpensive, and effective in treating Chemotherapy-induced mucositis. Clinically, radiation-induced mucositis can be also managed similarly to chemotherapy-induced mucositis. Mouthwashes that contain the tricyclic antidepressant doxepin have also been trialed in radiation-induced mucositis. In patients who receive mTOR inhibitor therapy, dexamethasone mouthwashes are effective at reducing the incidence and severity of mucositis with minimal adverse effects.
Some of the available treatment options in the market include Recombinant human keratinocyte growth factor-1 (palifermin) which reduces the incidence of WHO Grade 3 and 4 oral mucositis in patients with hematologic malignancies, Wecare’s Oralcare Rinse that helps in healing and repair of the oral mucosa, sodium fluoride that increases protection against cavities and Xylitol, which increases saliva production, RAM Pharma’s Acyclonine MUM, a dental powder used in the management of and for the temporary relief of symptoms, associated with acute and chronic oral mucosal ulcerations, oral mucositis, aphthous ulcers, and ulcerative lesions resulting from trauma, MuGard, a mucoadhesive oral wound rinse intended for the treatment of oral wounds such as mucositis, stomatitis, traumatic ulcers and aphthous ulcers, Episil that provides instant relief that is within five minutes post-application, and others. The dynamics of Oral mucositis market are anticipated to experience a positive shift in the coming years owing to the increase in the incidence of cancer and the expected entry of emerging therapies in the forecasted period (2024–2034).
Key players, such as Galera Therapeutics, EpicentRx, and others are evaluating their candidates in different stages of clinical development. They aim to investigate their products for the treatment of Oral mucositis.
Among the 7MM, the United States accounts for the highest Oral mucositis market size in 2023.
A few of the emerging Oral mucositis therapies that are anticipated to have a beneficial impact on the market landscape for oral mucositis, potentially leading to an expansion in market size include Avasopasem manganese, RRx-001, and MIT-001 among others.
There are limited specific treatment options for Oral Mucositis which opens a platform of new therapies to boost the Oral mucositis market.
Oral mucositis Drugs Uptake
This section focuses on the uptake rate of potential Oral mucositis drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging Oral mucositis therapies drug uptake in the report...
Oral mucositis Cancer Pipeline Activities
The Oral mucositis market report provides insights into Oral mucositis clinical trials within Phase III and Phase II stages. It also analyzes Oral mucositis companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Oral mucositis market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging Oral mucositis therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Professors and Researchers, Dentists, and Others.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as MD Anderson Cancer Center, Texas, UT Southwestern Medical Center in Dallas, Cancer Research UK Barts Centre in London, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Oral mucositis market trends.
| KOL Views |
| “A multimodal approach targeting the different domains involved in the pathogenesis of Oral Mucositis (OM) is the preferred treatment strategy. However, further research would lead to standard guidelines globally” |
| “All new agents are given prophylactically and are being evaluated in patients treated with concomitant chemoradiation therapy for head and neck cancer. The primary efficacy outcome for most trials is dependent on the assessment of mucositis using the scoring scale established years ago by the World Health Organization. The development activity for OM is robust with a diverse range of agents. New drug applications for effective therapeutic options for OM should be ready for review within the next few years.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging Oral mucositis therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Oral mucositis Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The Oral mucositis market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Oral mucositis Market Report
- The Oral mucositis market report covers a segment of key events, an executive summary, descriptive overview of Oral mucositis, explaining its causes, signs and symptoms, pathogenesis, and currently available Oral mucositis therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Oral mucositis market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Oral mucositis market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the Oral mucositis market.
Oral Mucositis Market Report Insights
- Oral mucositis Patient Population
- Oral mucositis Therapeutic Approaches
- Oral mucositis Pipeline Analysis
- Oral mucositis Market Size
- Oral mucositis Market Trends
- Oral mucositis Drugs Market
- Existing and future Oral mucositis Market Opportunity
Oral mucositis Market Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Oral mucositis Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Oral mucositis Therapies
- Oral mucositis Drugs Uptake
- Key Oral mucositis Market Forecast Assumptions
Oral mucositis Market Report Assessment
- Current Oral mucositis Treatment Practices
- Oral mucositis Unmet Needs
- Oral mucositis Pipeline Product Profiles
- Oral mucositis Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Oral mucositis Market Drivers
- Oral mucositis Market Barriers
FAQs
- What is the historical and forecasted Oral mucositis patient pool/patient burden in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What was the Oral mucositis total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Which treatment approaches will have a significant impact on the Oral mucositis market size?
- Is there any unexplored patient setting that can open the window for growth in the future?
- Which class is going to be the largest contributor to Oral mucositis market size in 2034?
- What are major changes in new treatment guidelines for treating Oral mucositis and what impact on future treatment landscape?
- What are the pricing variations among different geographies for approved Oral mucositis therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of Oral mucositis?
- How many companies are developing therapies for the treatment of Oral mucositis?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing Oral mucositis therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Oral mucositis therapies?
Reasons to buy Oral mucositis Market Forecast Report:
- The Oral mucositis market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Oral mucositis Market.
- Insights on patient burden/disease Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Patient-based forecast model which uses bottom-up forecasting techniques is accepted as a gold standard in pharma forecasting.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging Oral mucositis therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved Oral mucositis therapies, barriers to accessibility of expensive off-label Oral mucositis therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Get To Know Insights Through Relatdd Blogs From DelveInsight: