Abstract
Numerous treatments exist for COVID-19, the illness caused by SARS-CoV-2 virus, although most are not well established; among these are several small molecule antiviral agents. Intravenous remdesivir is an established treatment worldwide for inpatients and in some countries is also available for use in non-hospitalised high risk patients to prevent progression to severe disease and hospitalization. Oral molnupiravir and oral nirmatrelvir-ritonavir are also available in several countries to prevent progression to severe disease and hospitalization for high-risk outpatients. Many other antiviral small molecules that may have therapeutic potential are under investigation in clinical trials. This article provides a summary of key molecular targets, pharmacology and preliminary data on the efficacy and safety of small molecule antiviral agents being investigated for the treatment of COVID-19.
Similar content being viewed by others
Prevention better than COVID-19 cure
Much has been learned in the months since the severe acute respiratory syndrome (SARS)-CoV-2 coronavirus, which causes COVID-19, overwhelmed an unprepared world; however, pharmacological treatment options are still very limited [1, 2]. Despite the preferred preventative approach via vaccines, COVID-19 cases still abound [3]. Most cases are mild or asymptomatic, although still contagious [4]. For those more severely affected, COVID-19 treatment aims to reduce viral load and manage and dampen the overexuberant inflammatory response that causes the often fatal acute respiratory distress syndrome (ARDS) and myocarditis [1, 5].
Essential pharmacological treatment options include antiviral agents (to reduce viral load) as well as immunomodulators and biologics [2], particularly as it is the inflammatory response and release of proinflammatory cytokines (the “cytokine storm” characteristic of ARDS) rather than SARS-CoV-2 infection per se that is fatal [5]. Of interest, some antiviral agents also act as immunomodulators [1, 6].
The COVID-19 treatment pipeline includes both “repurposed” agents already approved or developed for other indications and new compounds [1]. Benefits of the former include known adverse drug events and ready availability, but the efficacy of most in COVID-19 has been lacklustre [2, 7].
Small molecules more accessible, if supply chain works
In a pandemic setting, reliable bulk manufacture of pharmacological agents is vital; however, it is likely that supply chain issues will arise [8]. Both small molecules and biologics are being studied in COVID-19 [3]. Overall, small molecules tend to be more accessible than biologics due to stability, oral formulation and lower cost (Table 1) [8,9,10].
This article reviews emerging small molecule antiviral (according to WHO ATC code or EPhMRA code) COVID-19 treatments up to mid-January 2022, with a focus on those furthest along the regulatory pathway. Discussion of the role of other small molecule classes, biologics and older immunomodulators like corticosteroids in the treatment of COVID-19 is outside the scope of this article.
Look at viral family resemblances and proteins...
Applying decades of knowledge of other coronaviruses to identify relevant, readily available drugs is seen as the fastest way to develop potential SARS-CoV-2 therapeutics [1]. The need for speed, however, should not overwhelm the need for evidence of good efficacy and safety [11].
SARS-CoV-2 is a large-genome, enveloped, positive-sense RNA virus of the genus Betacoronavirus and is closely related are SARS-CoV (for which SARS is named) and Middle East respiratory syndrome (MERS)-CoV [1, 12]. Both of these emerged in the twenty-first century to cause fatal human respiratory illness [1], but they mostly spread via nosocomial, not community, routes and pandemics were avoided [5]. SARS-CoV-2 also shares some features with unrelated viruses, e.g. HIV [13].
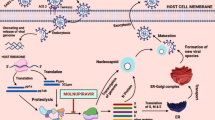
The SARS-CoV-2 genome codes for 4 structural proteins and 16 non-structural proteins (nsps [14]), all of which provide potential pharmaceutical antiviral targets (Table 2). Structural proteins are the three surface proteins [spike (S), membrane (M) and envelope (E)] and the two-domain, multifunctional nucleocapsid (N) protein [14]. The S protein, which has a glycan shield of uncertain significance, has S1 and S2 subunits and has been closely studied during vaccine development. The N protein, involved in RNA binding, is needed for effective viral replication [14]. Recent E protein studies indicate that its ability to affect cell polarity may correlate with viral virulence. Nsps 3-16 are similar across most coronaviruses, but nsp1 is not [15]. Some SARS-CoV-2 structural and non-structural proteins have a high degree of sequence similarity to those of SARS-CoV and MERS-CoV [1].
... and modify its lifecycle and immune effects
Like other viruses, SARS-CoV-2 infects humans cells by taking over host cell functions ranging from cell entry (to effect viral replication and assembly) to release from host cells (Table 2, [1, 13]). Small molecule antiviral treatments targeting these processes are accordingly broadly grouped into viral entry, replication and release inhibitors (Tables 2 and 3) [1], although the last are so far largely experimental in SARS-CoV-2 [1].
Most treatment aims to prevent or treat severe COVID-19 in high-risk patients. These include people who are older, obese or pregnant, as well as those with cancer (especially leukaemia or lymphoma), diabetes, or respiratory, cardiovascular and other comorbidities [6].
Investigations show that cytokines that drive the most severe illness include interleukins (ILs) 2, 6, 7 and 8, tumour necrosis factor (TNF)-⍺ and interferon (INF)-ɣ [5, 6]; for some of these cytokines, antagonists already exist [5]. Granulocyte-colony stimulating factor, INF-γ-inducible protein 10, macrophage inflammatory protein 1-α (MIP-1α), and monocyte chemoattractant protein-1 (MCP-1) are also of interest [5]. Clinical trials indicate that a low level of type 1 IFN is also a poor prognostic factor, as are elevated C-reactive protein and D-dimer levels [5, 16].
Mutating genes, resistance and safety matter
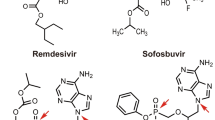
The potential for drug resistance, particularly for those to be used as monotherapy, needs to be considered when developing antivirals for the treatment of COVID-19. The propensity of SARS-CoV-2 for drug resistance is inversely proportional to its genetic stability [1]. Although its structural proteins are very stable [14], the mutations that do occur in the S1 subunit explain some of the increased infectivity of the ⍺, δ and omicron SARS-CoV-2 variants [13, 17]. Despite this, experience with SARS-CoV suggests resistance may not be a significant problem for viral entry inhibitors [1]. Resistance is also less of an issue with agents that directly target host cell, rather than viral, factors (Table 2). These agents may, helpfully, also reduce ARDS via immune modulation (Table 2). Nevertheless, resistance to remdesivir (an RdRp inhibitor) has been reported after emergence of an E802D mutation during treatment of an immune-deficient patient with COVID-19 (Table 3) [18].
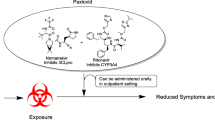
The choice of antiviral agent may depend on the potential for drug–drug interactions, particularly in patients who are being treated for underlying health issues and therefore may be at high risk of developing severe COVID-19 [19, 20]. Among the small molecule antivirals that are currently available for the treatment of COVID-19, nirmatrelvir-ritonavir [21,22,23] has numerous and complex drug–drug interactions (including with over-the-counter medicines and herbal supplements) because of the ritonavir component, which is required to achieve effective nirmatrelvir concentrations (Tables 3 and 4). For clinicians who are not experienced in prescribing ritonavir-boosted therapies, consultation with an expert should be considered [24]. In contrast, remdesivir [25, 26] and molnupiravir [27, 28] and their active metabolites do not inhibit or induce major drug metabolising enzymes and are not inhibitors of major drug transporters, so interaction with concomitant medications is unlikely (Tables 3 and 4).
Don’t gloss over gaps in pharmacology
Pharmacological features of several approved or almost-approved COVID-19 therapies are still being elucidated (Table 3), with ideal dosages sometimes unclear [4, 29, 30]. Investigations into several repurposed antivirals, such as favipravir [4, 29, 30], suggest the plasma and lung concentrations needed to treat COVID-19 may be higher than for influenza or HIV and perhaps closer to those required for Ebola treatment [4, 29]. Inhaled formulations of drugs used to treat COVID-19 infection can deliver the drug directly to the site of activity, avoid first-pass metabolism and have less systemic toxicity, so may be of benefit [31]. Clinically, the best constant plasma drug concentration may be at least the identified in vitro EC90; combinations of antivirals are also often preferred [30].
From a safety perspective, the ideal antiviral has an effective or inhibitory trough concentration (EC50 or IC50) well below the half-cytotoxic concentration [CC50] (Table 3) [4] and will not cause serious adverse drug events at the highest clinically effective dose (Table 4). Both the in vitro selectivity index and, especially for oral agents that will be used mostly at home, the clinical therapeutic index [4] are important. At this stage, clinical results (Table 4) are driving many decisions [11, 32].
Endpoints evolving in “learn-as-we-go” trials
Currently, the most prominent small molecule antivirals in clinical use are remdesivir [58], which has full approval for COVID-19 treatment in a number of countries (Tables 3 and 4) and molnupiravir and nirmatrelvir-ritonavir, which have either conditional approval or are in the regulatory preregistration or emergency use authorisation (EUA) phase of approval (Tables 3 and 4) [20]. Along with dexamethasone, remdesivir is often now the “standard of care” (SOC) comparator in clinical trials (Table 4). Lopinavir/ritonavir or hydroxychloroquine were common early SOCs [59], but newer meta-analyses and reviews do not support their use in COVID-19 [60,61,62]
Aside from some doubtful SOCs, comparing different agents is complex, as primary endpoints vary and have evolved with emerging understanding of COVID-19 [68]. In early trials, virological cure, preferably measured with the reverse transcription polymerase chain reaction (RT-PCR), was often the primary endpoint, but it was seldom achieved [60, 62]. Clinical primary endpoints are now more common (Table 4) and these may change during a trial (e.g. to day 29 from the original day 15 primary endpoint in the remdesivir ACCT-1 trial in severe disease [68]). Recent disappointments (e.g. for AT-527 [88] and eicosapentaenoic acid [7]) have resulted in a further rethink and/or a focus on narrower patient subgroups [89]. Drug dosages used in the treatment of patients with COVID-19 have also come under the spotlight [73, 90].
Newer COVID-19 studies are also taking place against a changing patient backdrop. Better understanding of COVID-19’s clinical course and consequent treatment protocols have contributed to significantly improved patient outcomes since the pandemic started [2, 91]. Between March 2020 and April 2021, COVID-19 mortality rates in selected US hospitals decreased from approximately 18% to 4% and length of stay from 12 to 7 days [92]. In-hospital patient demographics have also changed, with one US study reporting mild disease in almost half of those diagnosed with COVID-19 in early 2021, versus 36% during 2020 [93].
In terms of patient selection, criteria for mild, moderate and severe disease range from WHO and other ordinal scales to oxygen saturations (SpO2 %) and/or clinical descriptions [68, 69, 80]. As far as possible, disease severity in Table 4 is based on outpatient or hospital status and SpO2 as follows:
-
mild: non-hospitalised and sometimes asymptomatic, SpO2 ≥ 95%;
-
moderate: hospitalised but not needing active COVID-19 therapy or oxygen, SpO2 ≥ 94%;
-
moderate-severe: hospitalised, on supplemental oxygen but not mechanical ventilation, SpO2 ≥ 93%; or
-
severe-critical: requiring mechanical ventilation, SpO2 < 93%.
Two network meta-analyses [60, 62], which enable comparisons in the absence of head-to-head trials, assessed all-cause mortality, virological cure [60, 62], mechanical ventilation, hospital discharge and/or adverse drug events in 46 [60] and 222 [62] randomised, controlled trials reported in peer reviewed journals. Proxalutamide, nitazoxanide and remdesivir (Tables 3 and 4) improved some outcomes, as did the combination of remdesivir and baricitinib [60, 62].
A multitude of studies, but relatively few results
Hundreds of small molecule COVID-19 therapy studies are underway [1], with some preliminary efficacy and tolerability data now available (Table 5) and a range of registered trials in progress (Tables 4, 5 and 6).
Take home messages
-
Three small molecule antivirals, intravenous remdesivir, oral molnupiravir and oral nirmatrelvir-ritonavir are among the available COVID-19 treatments; other small molecule antivirals and immune modulators are urgently needed.
-
Small molecule antiviral therapies are practical pandemic options, as they are usually more easily manufactured, stable, orally available, less costly and may be repurposed from studies or use in viruses other that COVID-19, especially those closely related to SARS-CoV-2.
-
While COVID-19 treatments are a pressing need, approved medications should meet high efficacy and safety standards and there are many gaps in current knowledge.
-
Many small molecule antivirals show some promise in selected groups of COVID-19 patients, but large, randomised efficacy and safety studies of both monotherapy and combined antiviral agents are needed.
-
New small molecule antivirals have been designed with a high barrier for drug resistance; real-world data are required to confirm this. Combination therapy using small molecule antivirals with different mechanisms of action may be required to overcome drug resistance in the future.
-
Other administration routes for small molecule antivirals, including via inhalation, are a possibility.
References
Laws M, Surani YM, Hasan MM, et al. Current trends and future approaches in small-molecule therapeutics for COVID-19. Curr Med Chem. 2021;28(19):3803–24.
National Institutes of Health. Coronavirus Disease 2019 (COVID-19) treatment guidelines. 2021. https://www.covid19treatmentguidelines.nih.gov/. Accessed 5 Nov 2021.
Stanford Medicine. Race to a cure. 2021. https://racetoacure.stanford.edu/. Accessed 5 Nov 2021.
Joshi S, Parkar J, Ansari A, et al. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501–8.
Pillaiyar T, Wendt LL, Manickam M, et al. The recent outbreaks of human coronaviruses: a medicinal chemistry perspective. Med Res Rev. 2021;41(1):72–135.
Aldea M, Michot JM, Danlos FX, et al. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in Covid-19. Cancer Discov. 2021;11(6):1336–44.
Diaz R. PREPARE-IT 2: icosapent ethyl versus placebo in outpatients with Covid-19: the main results of PREPARE-IT 2 [abstract no. LBS.06]. In: #AHA21. 2021.
Makurvet FD. Biologics vs. small molecules: drug costs and patient access. Med Drug Discov. 2021;9:1075.
Ledford H. Dozens of coronavirus drugs are in development—what happens next? Nature. 2020;581(7808):247–8.
Nuventra. Points to consider in drug development of biologics and small molecules. 2020. https://www.nuventra.com/resources/blog/small-molecules-versus-biologics/. Accessed 4 Nov 2021.
Sidebottom DB, Smith DD, Gill D. Safety and efficacy of antivirals against SARS-CoV-2. BMJ. 2021;375:n2611.
Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–44.
Scudellari M. How the coronavirus infects cells—and why Delta is so dangerous. Nature. 2021;595(7869):640–4.
Troyano-Hernaez P, Reinosa R, Holguin A. Evolution of SARS-CoV-2 envelope, membrane, nucleocapsid, and spike structural proteins from the beginning of the pandemic to September 2020: a global and regional approach by epidemiological week. Viruses. 2021;13(2):243.
Khan MT, Irfan M, Ahsan H, et al. Structures of SARS-CoV-2 RNA-binding proteins and therapeutic targets. Intervirology. 2021;64(2):55–68.
Bruen C, Miller J, Schnaus M, et al. Auxora improves D-dimer levels in patients with severe COVID-19 pneumonia. Crit Care Med. 2021;49(1 Suppl.):134.
Syed AM, Ciling A, Khalid MM, et al. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. medRxiv. 2022. https://doi.org/10.1101/2021.12.20.21268048.
Gandhi S, Klein J, Robertson A, et al. De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. medRxiv. 2021. https://doi.org/10.1101/2021.11.08.21266069.
Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare providers. 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed 18 Jan 2022.
Burki TK. The role of antiviral treatment in the COVID-19 pandemic. Lancet. 2022. https://doi.org/10.1016/S2213-2600(22)00011-X.
European Medicines Agency. Paxlovid: EU summary of product characteristics. 2022. https://www.ema.europa.eu/en. Accessed 8 Feb 2022.
Medicines & Healthcare products Regulatory Agency. Paxlovid: summary of product characteristics. 2021. https://www.gov.uk/government/publications/regulatory-approval-of-paxlovid/summary-of-product-characteristics-for-paxlovid. Accessed 11 Jan 2022.
US Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for PAXLOVIDTM. 2021. https://www.fda.gov/media/155050/download. Accessed 11 Jan 2022.
National Institutes of Health. The COVID-19 Treatment Guidelines Panel's statement on potential drug-drug interactions between ritonavir-boosted nirmatrelvir (Paxlovid) and concomitant medications. 2021. https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-paxlovid-drug-drug-interactions/. Accessed 18 Jan 2022.
European Medicines Agency. Remdesivir (Veklury): EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 20 Dec 2021.
Gilead Sciences Inc. VEKLURY-remdesivir injection: US prescribing information. 2020. https://www.veklury.com/. Accessed 17 Jan 2022.
Medicines and Healthcare products Regulatory Agency. Regulatory approval of Lagevrio (molnupiravir). 2021. https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir. Accessed 21 Dec 2021.
Merck. Fact sheet for healthcare providers: emergency use authorization for molnupiravir. 2021. https://www.merck.com/eua/molnupiravir-hcp-fact-sheet.pdf. Accessed 21 Feb 2022.
Ison MG, Scheetz MH. Understanding the pharmacokinetics of favipiravir: implications for treatment of influenza and COVID-19. EBioMedicine. 2021;63:103204.
Pertinez H, Rajoli RKR, Khoo SH, et al. Pharmacokinetic modelling to estimate intracellular favipiravir ribofuranosyl-5’-triphosphate exposure to support posology for SARS-CoV-2. J Antimicrob Chemother. 2021;76(8):2121–8.
Eedara BB, Alabsi W, Encinas-Basurto D, et al. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutics. 2021;13(7):1077.
Zhao M, Zhang J, Li H, et al. Recent progress of antiviral therapy for coronavirus disease 2019. Eur J Pharmacol. 2021;890:173646.
Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–71.
Rosales R, McGovern BL, Rodriguez ML, et al. Nirmatrelvir, molnupiravir, and remdesivir maintain potent in vitro activity against the SARS-CoV-2 Omicron variant. bioRxiv. 2022. https://doi.org/10.1101/2022.01.17.476685.
Szemiel AM, Merits A, Orton RJ, et al. In vitro selection of remdesivir resistance suggests evolutionary predictability of SARS-CoV-2. PLoS Pathog. 2021;17(9):e1009929.
Vartak R, Patil SM, Saraswat A, et al. Aerosolized nanoliposomal carrier of remdesivir: an effective alternative for COVID-19 treatment in vitro. Nanomedicine (Lond). 2021;16(14):1187–202.
Li J, Zhang K, Wu D, et al. Liposomal remdesivir inhalation solution for targeted lung delivery as a novel therapeutic approach for COVID-19. Asian J Pharm Sci. 2021;16(6):772–83.
Sahakijpijarn S, Moon C, Koleng JJ, et al. Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics. 2020;12(11):1002.
Fujifilm Toyama Chemical Co. Ltd. AVIGAN tablets 200mg: prescribing information. 2014. https://www.cdc.gov.tw/File/Get/ht8jUiB_MI-aKnlwstwzvw. Accessed 17 Jan 2022.
Glenmark Pharmaceuticals Limited. Unlocking the treatment for mild to moderate Covid-19 in India [media release]. 20 Jun 2020. https://www.glenmarkpharma.com/sites/default/files/Glenmark-FabiFlu-Press-Brief.pdf.
Zhou S, Hill CS, Sarkar S, et al. beta-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021;224(3):415–9.
Wang Y, Zhong W, Salam A, et al. Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza. EBioMedicine. 2020;62(103125).
European Medicines Agency. Molnupiravir (Lagevrio): EU emergency use approval. 2021. https://www.ema.europa.eu/en/documents/referral/lagevrio-also-known-molnupiravir-mk-4482-covid-19-article-53-procedure-conditions-use-conditions_en.pdf. Accessed 21 Dec 2021.
Grobler J, Strizki J, Murgolo N, et al. Molnupiravir maintains antiviral activity against SARS-CoV-2 variants in vitro and in early clinical studies [abstract no. 543 and poster]. Open Forum Infect Dis. 2021;8(Suppl):S373.
Medicines and Healthcare products Regulatory Authority. First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA [media release]. 4 Nov 2021. https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra.
European Medicines Agency. Assessment report: use of molnupiravir for the treatment of COVID-19. 2021. https://www.ema.europa.eu/. Accessed 18 Jan 2022.
Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021. https://doi.org/10.1126/science.abl4784.
Pfizer Inc. Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study [media release]. 5 Nov 2021. https://www.pfizer.com/.
Pfizer Inc. Pfizer reports second-quarter 2021 results [media release]. 4 July 2021. https://s21.q4cdn.com/317678438/files/doc_financials/2021/q2/Q2-2021-PFE-Earnings-Release.pdf.
Pfizer Inc. Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death [media release]. 14 Dec 2021. https://www.pfizer.com/.
Greasley SE, Noell S, Plotnikova O, et al. Structural basis for nirmatrelvir in vitro efficacy against the Omicron variant of SARS-CoV-2. bioRxiv. 2022. https://doi.org/10.1101/2022.01.17.476556.
Rai DK, Yurgelonis I, McMonagle P, et al. Nirmatrelvir, an orally active Mpro inhibitor, is a potent inhibitor of SARS-CoV-2 variants of concern. bioRxiv. 2022:2022.01.17.476644.
Kintor Pharmaceutical Limited. Kintor Pharma provides update on one of its three multi-regional phase 3 trials of proxalutamide for COVID-19 [media release]. 27 Dec 2021. https://en.kintor.com.cn/.
Li H, Ran R, Jiang H, et al. Evaluation of safety, pharmacokinetics and pharmacodynamics of proxalutamide (GTO918), a potent androgen receptor (AR) blocker, in patients with metastatic brest cancer (mBC): phase 1 dose escalation trial [abstract no. P2-17-05]. Cancer Res. 2020;80(4 Suppl).
Romark Pharmaceuticals. Alinia (nitazoxanide) oral tablets and suspension: US prescribing information. 2005. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021818lbl.pdf. Accessed 5 Nov 2021.
Romark Pharmaceuticals. Romark initiates phase 3 clinical trials of NT-300 for COVID-19 [media release]. 27 April 2020. https://www.romark.com/.
Romark Pharmaceuticals. Romark announces initial results of phase 3 clinical trial of NT-300 tablets for the treatment of COVID-19 [media release]. 14 Apr 2021. https://www.romark.com/.
Vitiello A, Troiano V, La Porta R. What will be the role of molnupiravir in the treatment of COVID-19 infection? Drugs Ther Perspect. 2021;37(12):579–80.
Shinkai M, Tsushima K, Tanaka S, et al. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial. Infect Dis Ther. 2021;10(4):2489–509.
Cheng Q, Chen J, Jia Q, et al. Efficacy and safety of current medications for treating severe and non-severe COVID-19 patients: an updated network meta-analysis of randomized placebo-controlled trials. Aging (Milano). 2021;13:1–37.
Gil Martinez V, Avedillo Salas A, Santander BS. Antiviral therapeutic approaches for SARS-CoV-2 infection: a systematic review. Pharmaceuticals (Basel). 2021;14(8):736.
Zhang C, Jin H, Wen YF, et al. Efficacy of COVID-19 treatments: a Bayesian network meta-analysis of randomized controlled trials. Front Public Health. 2021;9:729559.
Gilead Sciences. Fact sheet for health care providers emergency use authorization (EUA) oF VEKLURY® (remdesivir). 2022. https://www.gilead.com/remdesivir. Accessed 25 Jan 2022.
Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2116846.
Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383(19):1827–37.
Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–57.
Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384(6):497–511.
Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383(19):1813–26.
Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–78.
Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384(9):795–807.
Zhang D, Du G, Du R, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020;395(10236):1569–78.
US National Library of Medicine. ClinicalTrials.gov. 2022. https://clinicaltrials.gov/. Accessed 17 Jan 2022.
Pertinez H, Rajoli RK, Khoo SH, et al. Pharmacokinetic modelling to estimate intracellular favipiravir ribofuranosyl-5'-triphosphate exposure to support posology for SARS-CoV-2. medRxiv. 2021.
Corritori S, Yakubova E, Ivashchenko A, et al. Multicenter, open-labeled efficacy study of avifavir in patients with COVID-19. Top Antivir Med. 2021;29(1):138.
Manabe T, Kambayashi D, Akatsu H, et al. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):489.
Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. Avifavir for treatment of patients with moderate Coronavirus Disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73(3):531–4.
Chen C, Huang J, Yin P, et al. Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: a prospective, multicenter, open-label, randomized controlled clinical trial. Front Pharmacol. 2021;12(683296).
Doi Y, Hibino M, Hase R, et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/aac.01897-20.
Zhao H, Zhang C, Zhu Q, et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: a multicenter, open-label, randomized trial. Int Immunopharmacol. 2021;97:107702.
Khamis F, Al Naabi H, Al Lawati A, et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis. 2021;102:538–43.
Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2021.
Caraco Y, Crofoot GE, Moncada PA, et al. Phase 2/3 trial of molnupiravir for treatment of Covid-19 in nonhospitalized adults. NEJM Evidence. 2021. https://doi.org/10.1056/EVIDoa2100043.
Arribas JR, Bhagani S, Lobo SM, et al. Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19. NEJM Evid. 2021. https://doi.org/10.1056/EVIDoa2100044.
Painter WP, Holman W, Bush JA, et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother. 2021;65(5):e02428-e2520.
Kintor Pharmaceutical Limited. Kintor Pharmaceutical receives emergency use authorization for proxalutamide for the treatment of COVID-19 in Paraguay [media release]. 16 July 2021. https://en.kintor.com.cn/.
Cadegiani FA, do Nascimento Fonseca D, McCoy J, et al. Efficacy of proxalutamide in hospitalized COVID-19 patients: a randomized, double-blind, placebo-controlled, parallel-design clinical trial. medRxiv. 2021. https://doi.org/10.1101/2021.06.22.21259318.
Cadegiani FA, Zimerman RA, Fonseca DN, et al. Final results of a randomized, placebo-controlled, two-arm, parallel clinical trial of proxalutamide for hospitalized COVID-19 patients: a multiregional, joint analysis of the Proxa-Rescue AndroCoV trial. Cureus. 2021;13(12):e20691.
Atea Pharmaceuticals. Atea Pharmaceuticals provides update and topline results for phase 2 MOONSONG trial evaluating AT-527 in the outpatient setting [media release]. 19 Oct 2021. https://ir.ateapharma.com/news-releases/news-release-details/atea-pharmaceuticals-provides-update-and-topline-results-phase-2.
Amarin Corporation. Amarin reports overview of latest clinical research evaluating VASCEPA®/VAZKEPA (icosapent ethyl) and eicosapentaenoic acid (EPA) presented at ESC Congress 2021, organized by the European Society of Cardiology [media release]. 31 Aug 2021. https://investor.amarincorp.com/news-releases/news-release-details/amarin-reports-overview-latest-clinical-research-evaluating.
Rajoli RKR, Pertinez H, Arshad U, et al. Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis. Br J Clin Pharmacol. 2021;87(4):2078–88.
Immunic Inc. Immunic, Inc. announces that oral treatment IMU-838 shows evidence of clinical activity in moderate COVID-19 in phase 2 CALVID-1 trial [media release]. 17 Feb 2021. https://imux.com/
National Center for Health Statistics. In-hospital mortality among hospital confirmed COVID-19 encounters by week from selected hospitals. 2021. https://www.cdc.gov/nchs/covid19/nhcs/hospital-mortality-by-week.htm. Accessed 5 Nov 2021.
David Sweig for the Atlantic Monthly. Our most reliable pandemic number is losing meaning. 2021. https://www.theatlantic.com/health/archive/2021/09/covid-hospitalization-numbers-can-be-misleading/620062/. Accessed 5 Nov 2021.
Wilkinson T, Gausdal G, McCracken N, et al. Bemcentinib, an oral AXL kinase inhibitor, results in lower mortality compared to standard of care (steroids with or without remdesivir) in hospitalised patients with COVID-19. Two randomised phase 2 studies: BGBC020 and ACCORD2. Online presentation at 31st ECCMID. 2021. https://www.bergenbio.com/wp-content/uploads/2021/07/ECCMID-2021-LB-oral-Bemcentinib-12jul2021-.pdf. Accessed 17 Jan 2022.
BerGenBio. BerGenBio announces top line data from phase III trial assessing bemcentinib in hospitalised Covid-19 patients [media release]. 18 May 2021. https://www.bergenbio.com/.
RedHill Biopharma. RedHill Biopharma reports further analysis of phase 2/3 data including a 62% reduction in mortality with oral opaganib in moderately severe COVID-19 patients [media release]. 4 Oct 2021. https://www.redhillbio.com/.
Veru Pharma. Sabizabulin for COVID-19: (VERU-111). 2021. https://verupharma.com/pipeline/sabizabulin-for-covid-19/. Accessed 5 Nov 2021.
Veru Pharma. Veru reports positive phase 2 clinical results of VERU-111 in hospitalized COVID-19 patients at high risk for acute respiratory distress syndrome [media release]. 8 Feb 2021. https://verupharma.com/.
Zhou XJ, Horga A, Morelli G, et al. High lung levels of active triphosphate predicted with oral AT-527 in COVID patients [abstract no. 364]. Top Antivir Med. 2021;29(1):127.
Good SS, Westover J, Jung KH, et al. AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for treatment of COVID-19. Antimicrob Agents Chemother. 2021;65(4):e02479-e2520.
Miller J, Bruen C, Schnaus M, et al. Auxora versus standard of care for the treatment of severe or critical COVID-19 pneumonia: results from a randomized controlled trial. Crit Care. 2020;24(1):502.
Senhwa Biosciences. Senhwa presents positive initial data from phase 2 clinical trial of silmitasertib (CX-4945) in moderate COVID-19 patients at the ISIRV-WHO conference [media release]. 20 Oct 2021. https://www.senhwabio.com/.
Sorrento Therapeutics. Sorrento announces encouraging results from two phase 2 studies of abivertinib for treatment of hospitalized severe COVID-19 patients [media release]. 27 Oct 2021. https://investors.sorrentotherapeutics.com/.
Bakovic A, Risner K, Bhalla N, et al. Brilacidin, a COVID-19 drug candidate, exhibits potent in vitro antiviral activity against SARS-CoV-2. bioRxiv. 2020. https://doi.org/10.1101/2020.10.29.352450.
Hu Y, Jo H, DeGrado WF, et al. Brilacidin, a COVID-19 drug candidate, demonstrates broad-spectrum antiviral activity against human coronaviruses OC43, 229E and NL63 through targeting both the virus and the host cell. bioRxiv. 2021. https://doi.org/10.1101/2021.11.04.467344.
Innovation Pharmaceuticals. Innovation Pharmaceuticals announces topline results from phase 2 clinical trial of brilacidin for COVID-19 [media release]. Nov 11 2021. http://www.ipharminc.com/.
Innovation Pharmaceuticals. Innovation Pharmaceuticals conducting full data analysis of phase 2 brilacidin COVID-19 trial results to support brilacidin’s potential inclusion in government-sponsored COVID-19 trials [media release]. Nov 12 2021. http://www.ipharminc.com/.
Innovation Pharmaceuticals. Innovation Pharmaceuticals analyzing full dataset for its brilacidin COVID-19 clinical trial; company evaluating new pipeline opportunities for 2022 [media release]. Dec 7 2021. http://www.ipharminc.com/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
C Fenton, a contracted employee of Adis International Ltd/Springer Nature, and S Keam, a salaried employee of Adis International Ltd/Springer Nature, declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: S.A. Antoniu, ‘Grigore T. Popa’ University of Medicine and Pharmacy, Department of Medicine II, Pulmonary Disease University Hospital, Iaşi, Romania; S. Mirkov, University of Otago School of Pharmacy, Dunedin, New Zealand; A Vitiello, USL Umbria 1 Pharmaceutical Department, Italy.
Rights and permissions
About this article
Cite this article
Fenton, C., Keam, S.J. Emerging small molecule antivirals may fit neatly into COVID-19 treatment. Drugs Ther Perspect 38, 112–126 (2022). https://doi.org/10.1007/s40267-022-00897-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00897-8