Precision-Guided Dosing
![[LOGO] PredictrPK (R) [LOGO] PredictrPK (R)](/wp-content/uploads/2023/01/LOGO-PredictrPK-R.png)
![[Page Header] PPK main page 20230705 [Page Header] PPK main page 20230705](https://prometheuslabs.com/wp-content/uploads/2023/07/Page-Header-PPK-main-page_20230705.png)
Biologic dosing is not one size fits all
The unpredictable pharmacokinetic variability of biologic therapies amongst individual
inflammatory bowel disease (IBD) patients makes it extremely difficult to achieve
therapeutic targets quickly and precisely.1
• Up to 40% of patients who start an anti-TNF will lose response in year one2
• Over 50% will require dose optimization during their treatment3
• Current tests provide a single point-in-time snapshot on drug levels, but don’t provide insights on dosage or interval to achieve targets.
Conventional dosing poses many challenges for providers and patients alike.4-6
• Suboptimal therapeutic levels
• Potential loss of response
• Inefficient care
• Negative impact on quality of life
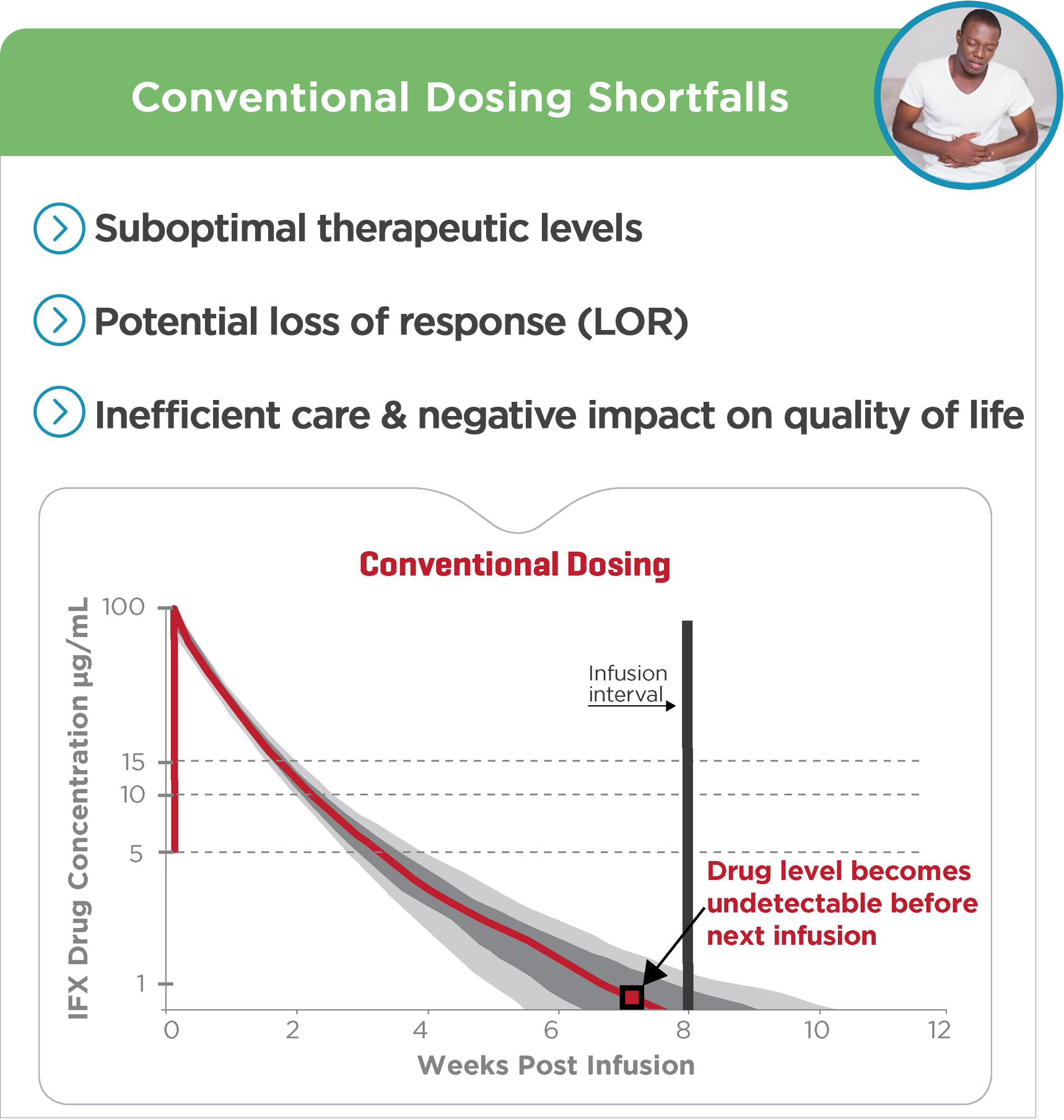
Conventional approaches to drug monitoring can lead to unoptimized therapy
resulting in inefficient care, disease recurrence and potential treatment failure
Control the trajectory of therapeutic drug levels with precision-guided care
PredictrPK®, available exclusively from Prometheus Laboratories, offers individualized, actionable evidence to expediently fine-tune infliximab (IFX) and adalimumab (ADA) dosing in support of durable response and sustained clinical remission. These paradigm shifting tests help guide biologic optimization and aid in controlling the trajectory of therapeutic drug levels and achieving treatment targets.
![[Bugs] No Clearance 20230620 [Bugs] No Clearance 20230620](https://prometheuslabs.com/wp-content/uploads/2023/07/Bugs-No-Clearance_20230620.png)
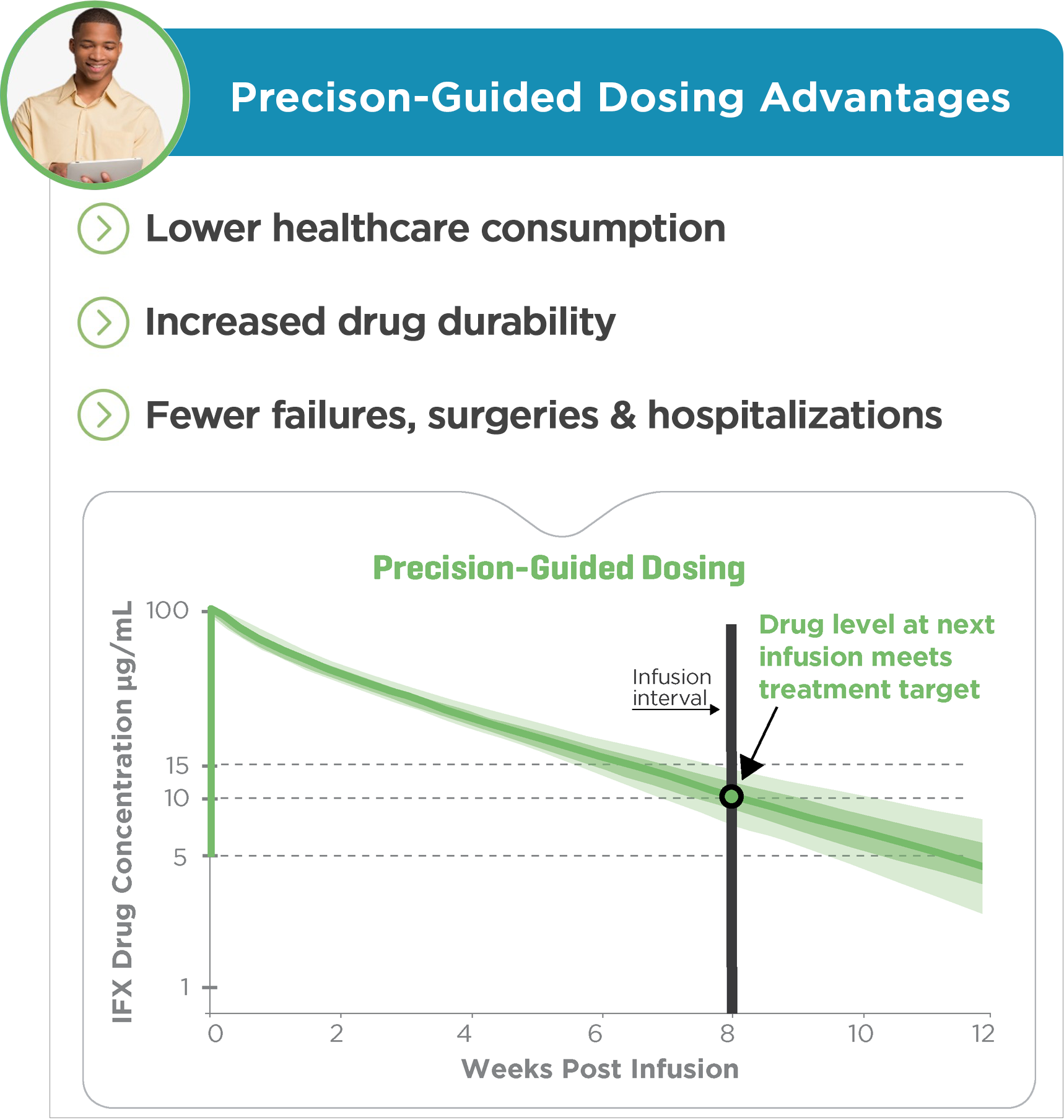
PredictrPK provides objective, individualized predictions of optimal biologic dosing for IFX and ADA that empowers providers to precisely:
• Accelerate treatment optimization supporting drug persistence
• Increase certainty of achieving therapeutic targets
• Ensures patients get the most from infliximab therapy
• Improved patient confidence and satisfaction
Evidence-based outcomes of dosing optimization:4-9
• Durability of response
• Improved remission rates
• Reduced healthcare consumption
• Improved quality of life and efficient care
PredictrPK ADA was validated in a cohort of adult Crohn’s disease patients receiving adalimumab (ADA) therapy. PredictrPK ADA can be utilized during steady-state maintenance, after 8 weeks of continuous, on-time, ADA therapy. Serum samples can be collected anytime within the prescribed dosing interval. PredictrPK IFX Induction and PredictrPK IFX Maintenance were validated in cohorts of adult and pediatric IBD patients receiving infliximab (IFX) therapy. Induction testing is available ≤3 days prior to induction infusion 3. Maintenance testing is available ≤3 days prior to 14 weeks of continuous, on-time IFX therapy or ≥20 days after any regular maintenance infusion, up to and including the next trough. PredictrPK IFX and PredictrPK ADA can be used on patients taking IFX and ADA originators or their biosimilars including patients on combination therapy with an immunomodulator or another therapeutic agent
References
- Vermeire et al. Clin Gastroenterol Hepatol. 2020; 18:1291–1299.
- Ben-Horin et al. Autoimmun Rev. 2014; 13:24-30.
- O’Donnell et al. J Crohns Colitis. 2015; 9:830–836.
- Negoescu et al. Inflamm Bowel Dis. 2020; 26:103-111.
- Papamichael et al. J Crohns Colitis. 2018; 12:804-810.
- Papamichael et al. Clin Gastroenterol Hepatol. 2017; 15:1580-1588.
- Strik et al. Scand J Gastroenterol. 2021; 56:145-154.
- Dubinsky et al. Inflamm Bowel Dis. 2022; 28:1375-1385.
- Primas et al. J Clin Med. 2022; 11:3316.
- C Law et al. J Crohn’s and Colitis. 2024; 18(S1): i1102–i110.




