Setting New
Standards for
Venous Care
Setting New
Standards for
Venous Care
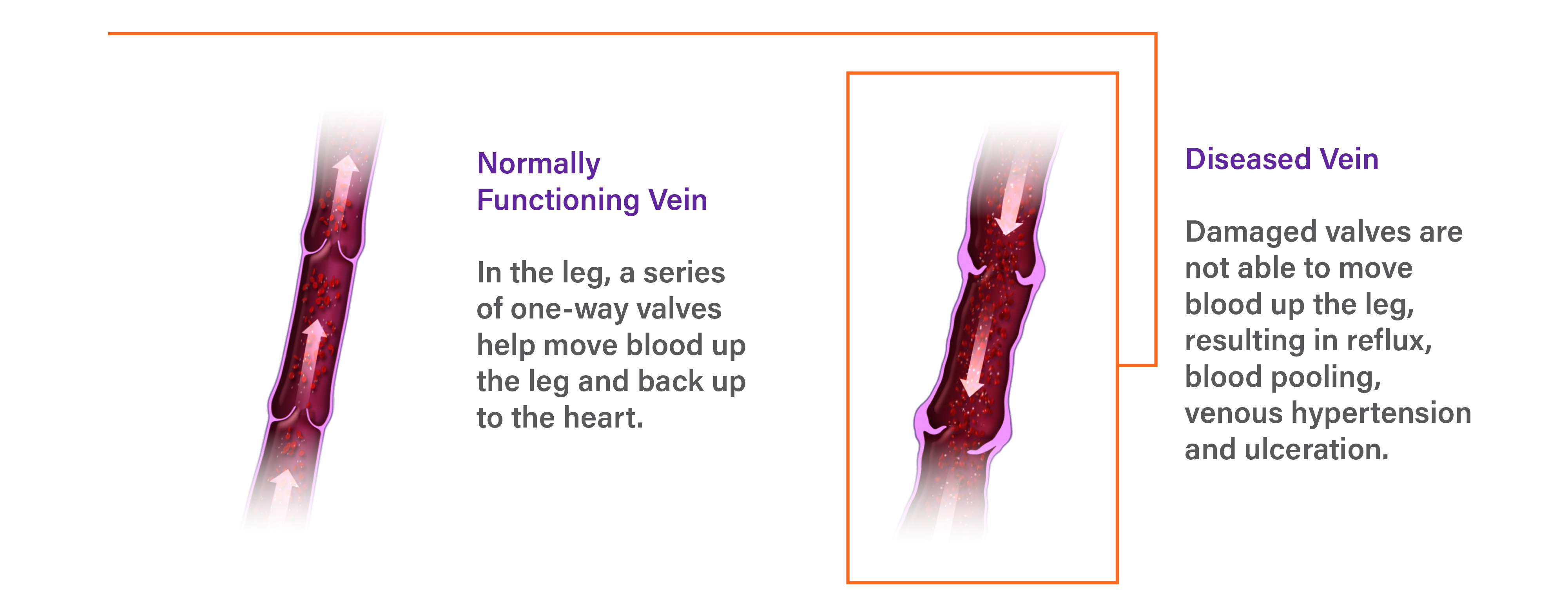
What is Chronic Venous Insufficiency (CVI)?
Insufficient blood is returned to the heart and lungs due to damaged valves in the veins of the leg.
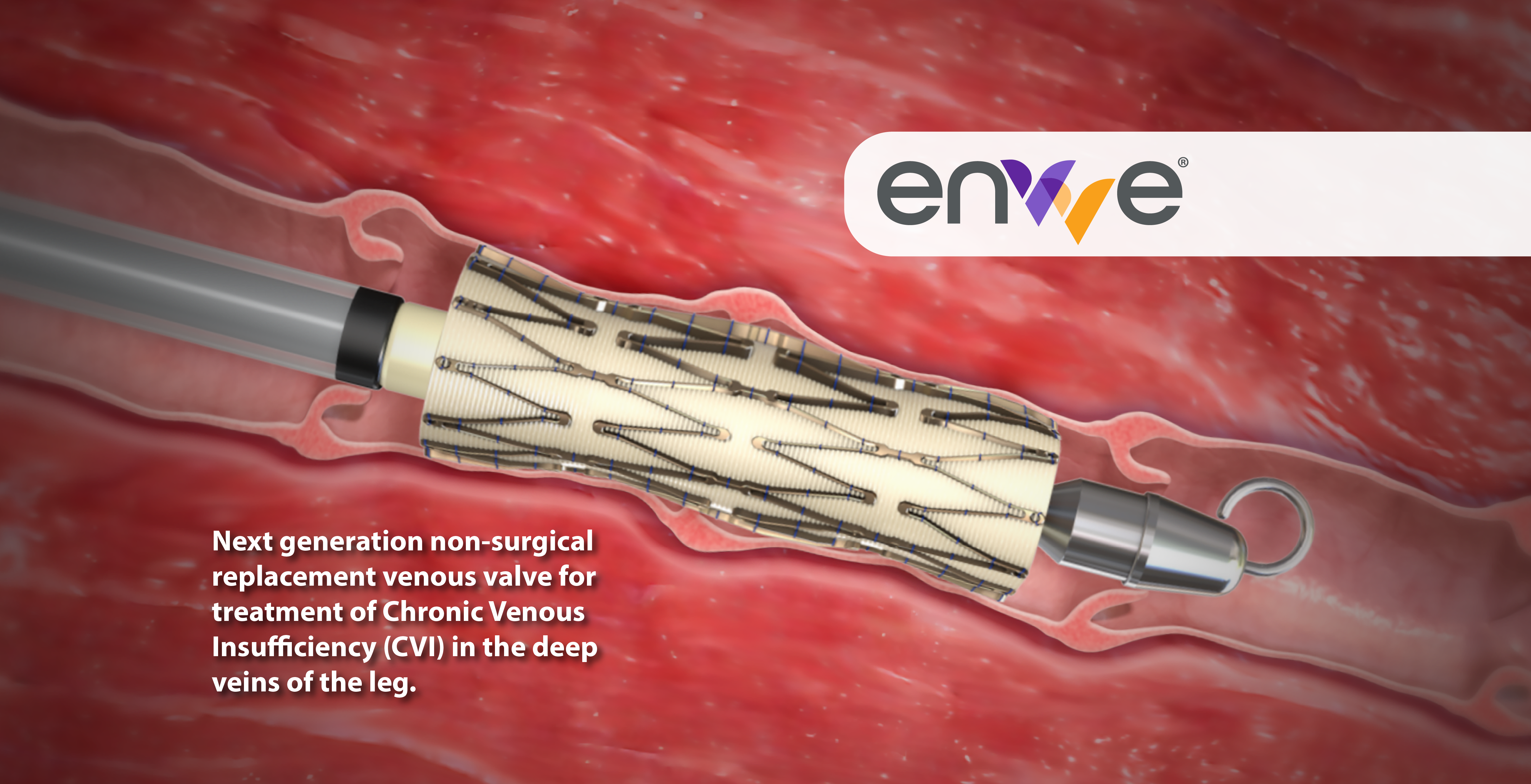
First-in-class surgical replacement venous valve for treatment of Chronic Venous Insufficiency (CVI) in the deep veins of the leg.
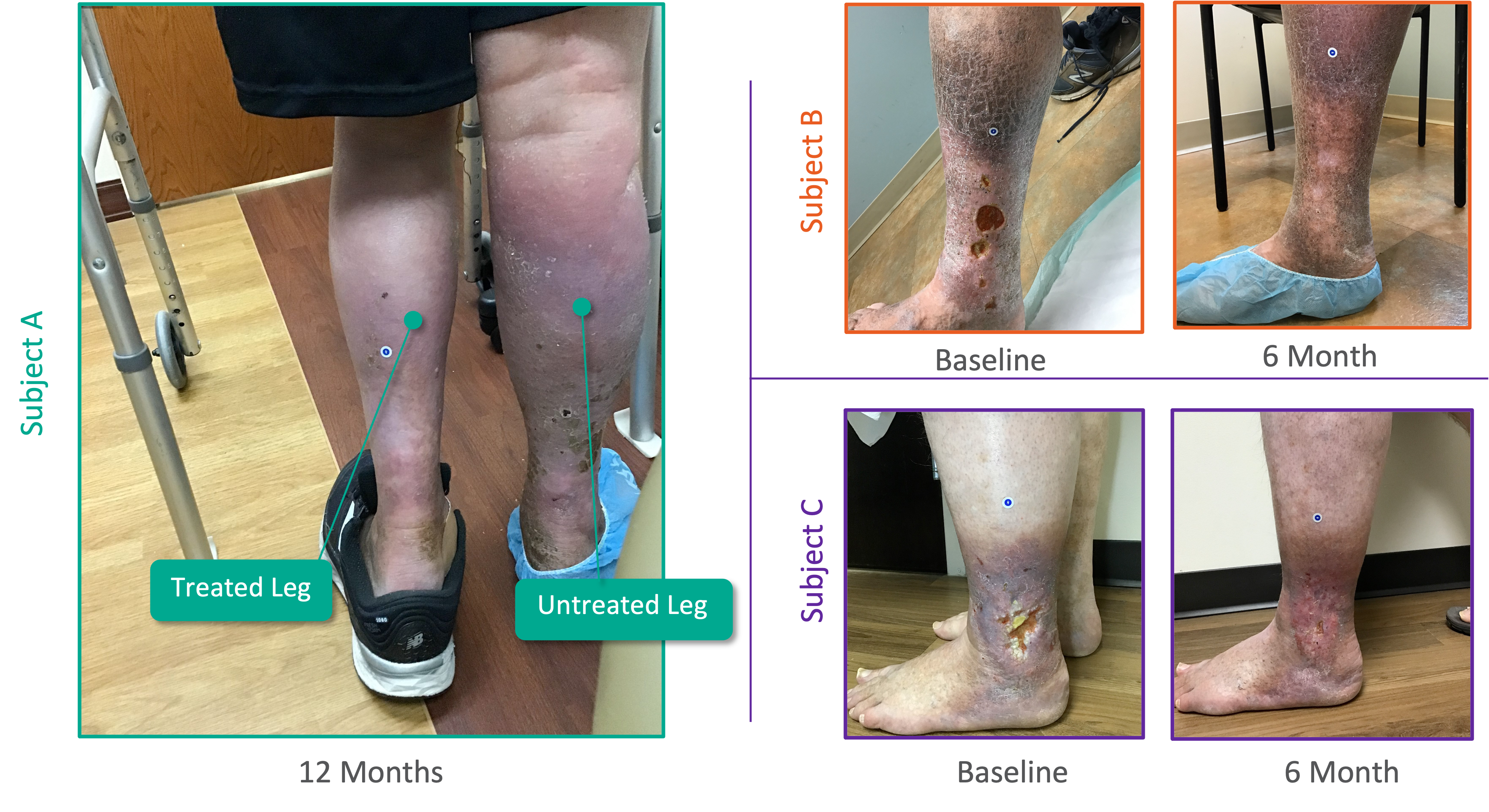
Patient Experience From Pivotal SAVVE® Trial

Initial Positive 11-Month Topline Efficacy Data From SAVVE Trial*
- Overall 8.46 Average Revised Venous Clinical Severity Score (rVCSS) Improvement Per Patient for Patients Showing Clinical Meaningful Benefit (rVCSS Improvement ≥ 3 Points) including:
- 9.29 Points for Patients at the Two-Year Milestone
- 8.08 Points for Patients at the One-Year Milestone
- 8.71 Points for Patients at the Six-Month Milestone
- 72% of the Study Patients Showing Clinical Meaningful Benefit from the VenoValve at a Weighted Average of 11 Months Post Surgery
- 94% of VenoValve Study Patients Showing Clinical Improvement at a Weighted Average of Eleven Months Post Surgery (rVCSS Improvement ≥ 1 point)
* Data compared to baseline
Latest News
Stock Data
The VenoValve® and enVVe® are investigational medical devices currently in development. Neither device is approved or cleared for any indication in any market. The VenoValve® is only available for use in the United States in pre-market clinical studies.