Affordable, accessible vaccines & antibodies
We are a nonprofit scientific research organization that develops vaccines and antibodies for HIV, tuberculosis, and emerging infectious diseases.
About usOur approach
We take a full-circle approach to product development, from the grassroots level to the lab to legislative bodies.
Mission
To translate scientific discoveries into affordable, globally accessible public health solutions.
Vision
A world where all people have equitable access to innovative vaccines and therapeutics.

Our health areas
- HIV
- Tuberculosis
- Lassa virus
- Marburg virus
- Sudan virus
HIV
The challenge
HIV/AIDS remains one of the deadliest infectious diseases, with 1.3 million people acquiring HIV in 2023. Given the complexities of HIV, traditional approaches to vaccine development have so far failed to result in a vaccine that provides protection against HIV. However, a vaccine is still needed to bring a true end to the HIV pandemic. Source: UNAIDS
Our solution
IAVI scientists and our collaborators are developing next-generation HIV vaccines to address the challenges of HIV vaccine design. Together, we have pioneered promising new vaccination strategies and the discovery of broadly neutralizing antibodies (bnAbs) against HIV. IAVI and our partners are developing HIV bnAbs to prevent HIV acquisition while we advance vaccine candidates.
Tuberculosis
The challenge
Tuberculosis (TB) is the world’s leading infectious disease killer. In 2023, 10.8 million people fell ill with TB and 1.25 million people died of TB disease. The only available TB vaccine is the century-old Bacille Calmette-Guérin, or BCG. While this vaccine has efficacy in protecting against severe TB disease in infants and young children, it is largely ineffective in adolescents and adults, among whom most transmission and disease occurs. Source: WHO
Our solution
IAVI works across our global hubs with a diverse network of partners to advance the most promising TB vaccine candidates from discovery through clinical trials, and eventually, to post-licensure access. Our work extends to policy and advocacy initiatives that support TB vaccine development and access in regions where new vaccines are needed most.
Lassa virus
The challenge
Lassa virus is an arenavirus that causes Lassa fever, an acute viral hemorrhagic illness endemic to West Africa that causes significant annual outbreaks of disease. Lassa fever is difficult to diagnose, and surveillance data is limited. Current estimates range from 300,000 to 500,000 cases and 5,000 related deaths each year. No licensed vaccines for Lassa fever exist. Source: WHO
Our solution
IAVI is developing a single-dose vaccine candidate for Lassa that is based on a recombinant vesicular stomatitis virus (rVSV) vector. This technology is similar to that underlying an approved Ebola Zaire virus vaccine. In 2024, IAVI’s Lassa vaccine candidate became the first Lassa vaccine to enter Phase 2 clinical trials, launched in three West African countries. This trial builds on a Phase 1 clinical trial that showed the candidate vaccine was well tolerated and immunogenic over a wide dose range. IAVI also conducts Lassa epidemiological studies, modeling, and community engagement to support future vaccine access.
Marburg virus
The challenge
Marburg virus is a filovirus and the causative agent of Marburg virus disease (MVD), which has a case fatality ratio of up to 88%. Marburg virus has the capacity to cause outbreaks with high fatality rates and is a potential bioterror threat. No vaccines or antiviral treatments are approved for MVD. A Marburg vaccine is urgently needed to respond to future outbreaks. Source: WHO
Our solution
IAVI is developing a vaccine candidate for Marburg that is based on a recombinant vesicular stomatitis virus (rVSV) vector. This technology is similar to that underlying an approved Ebola Zaire virus vaccine, which is now approved by the U.S. FDA and registered for use in several African countries. Preclinical data demonstrates that IAVI’s Marburg vaccine candidate is highly protective in an animal model with one dose.
Sudan virus
The challenge
Sudan virus (SUDV) is a filovirus and causes outbreaks of Ebola disease, most recently in 2022 in Uganda. Case fatality rates of Ebola disease caused by SUDV have varied from 41% to 100% in past outbreaks. The licensed vaccine for Ebola Zaire virus does not provide cross protection against the Sudan strain. A SUDV vaccine is urgently needed to prevent and respond to future outbreaks. Source: WHO
Our solution
IAVI is developing a vaccine candidate for SUDV that is based on a recombinant vesicular stomatitis virus (rVSV) vector similar to the technology underlying an approved Ebola Zaire virus vaccine. During an Ebola outbreak starting in January 2025 in Uganda, the WHO prioritized IAVI’s SUDV vaccine candidate for a ring vaccination trial and delivered the first vaccinations just four days after the outbreak was declared as part of a comprehensive public health response.
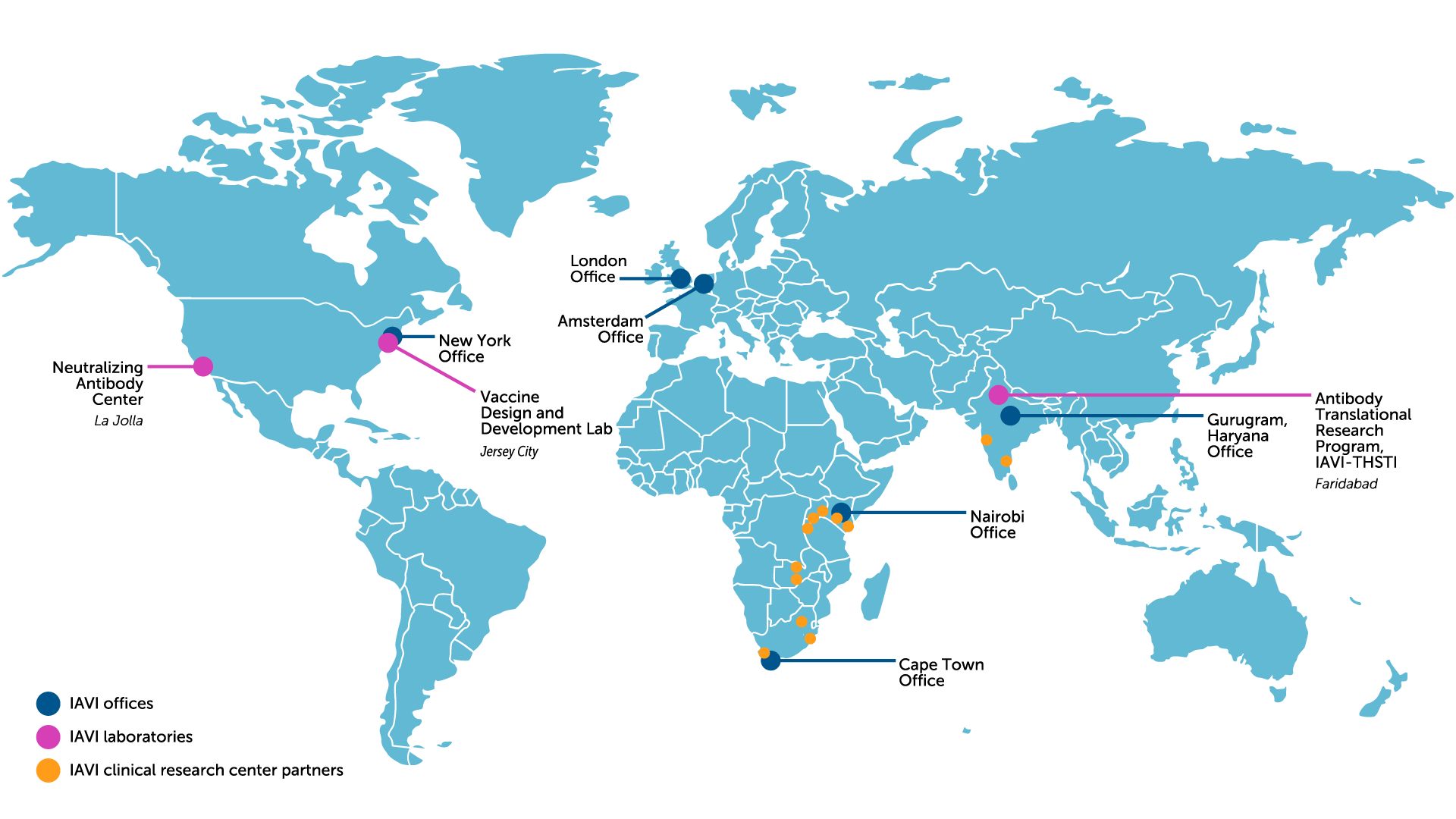
Our locations
IAVI Africa
- Cape Town Office, South Africa
- Nairobi Office, Kenya
IAVI Europe
- Amsterdam Office, The Netherlands
- Human Immunology Lab, London, U.K.
IAVI India
- Gurugram, Haryana Office
- Antibody Translational Research Program, IAVI-THSTI, Faridabad
IAVI U.S.
- New York Office
- Vaccine Design & Development Lab, New York
- Neutralizing Antibody Center, California
Recent news & media


IAVI announces termination of the PEPFAR-funded ADVANCE program and staff reductions
February 27, 2025
A tragic reminder of the power of vaccination
March 17, 2025
Meet the women at the helm of IAVI’s Lassa program
March 06, 2025
IAVI and Biofabri/Zendal announce first vaccinations in the IMAGINE clinical trial, a large-scale safety and efficacy trial of the tuberculosis vaccine candidate MTBVAC
February 26, 2025